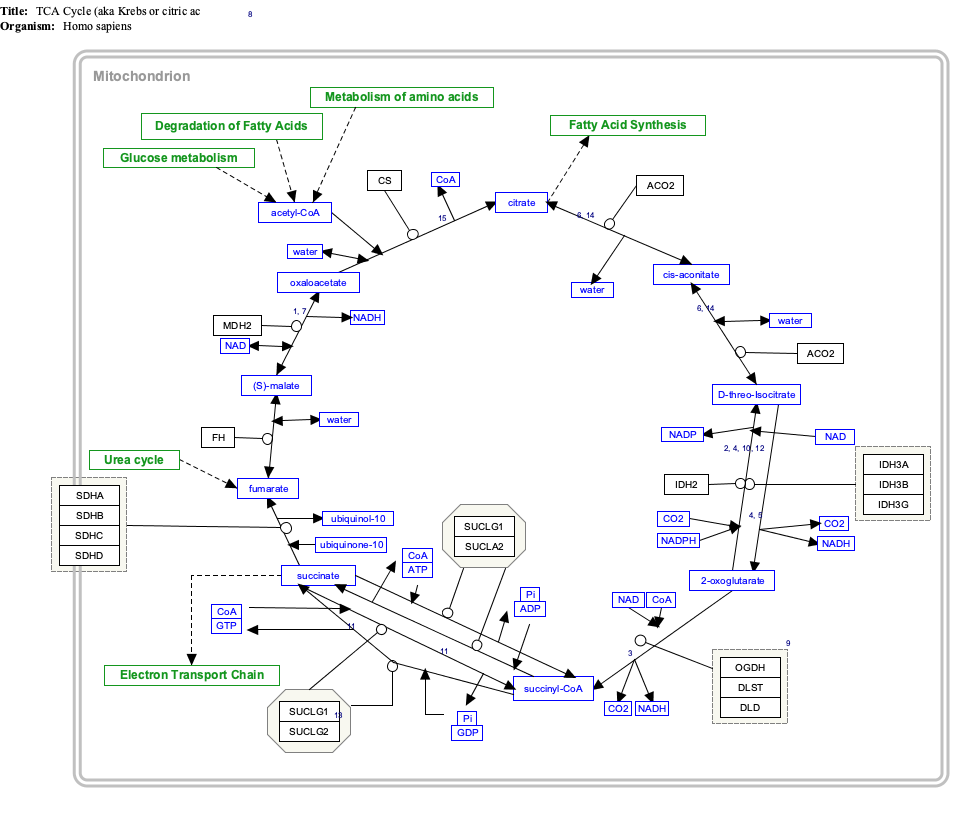
Citric acid cycle

The citric acid cycle—also known as the Krebs cycle, Szent–Györgyi–Krebs cycle or the TCA cycle (tricarboxylic acid cycle)
The name of this metabolic pathway is derived from the citric acid (a tricarboxylic acid, often called citrate, as the ionized form predominates at biological pH[6]) that is consumed and then regenerated by this sequence of reactions to complete the cycle. The cycle consumes acetate (in the form of acetyl-CoA) and water, reduces NAD+ to NADH, releasing carbon dioxide. The NADH generated by the citric acid cycle is fed into the oxidative phosphorylation (electron transport) pathway. The net result of these two closely linked pathways is the oxidation of nutrients to produce usable chemical energy in the form of ATP.
In
For each pyruvate molecule (from glycolysis), the overall yield of energy-containing compounds from the citric acid cycle is three NADH, one FADH2, and one GTP.[7]
Discovery
Several of the components and reactions of the citric acid cycle were established in the 1930s by the research of
Overview
This section needs additional citations for verification. (August 2022) |
The citric acid cycle is a
One of the primary sources of acetyl-CoA is from the breakdown of sugars by glycolysis which yield pyruvate that in turn is decarboxylated by the pyruvate dehydrogenase complex generating acetyl-CoA according to the following reaction scheme:
The product of this reaction, acetyl-CoA, is the starting point for the citric acid cycle. Acetyl-CoA may also be obtained from the oxidation of fatty acids. Below is a schematic outline of the cycle:
- The acetylgroup from acetyl-CoA to the four-carbon acceptor compound (oxaloacetate) to form a six-carbon compound (citrate).
- The citrate then goes through a series of chemical transformations, losing two anabolism, they might not be lost, since many citric acid cycle intermediates are also used as precursors for the biosynthesis of other molecules.[12]
- Most of the electrons made available by the oxidative steps of the cycle are transferred to NAD+, forming NADH. For each acetyl group that enters the citric acid cycle, three molecules of NADH are produced. The citric acid cycle includes a series of redox reactions in mitochondria.[clarification needed][13]
- In addition, electrons from the succinate oxidation step are transferred first to the Complex III.
- For every NADH and FADH2 that are produced in the citric acid cycle, 2.5 and 1.5 ATP molecules are generated in oxidative phosphorylation, respectively.
- At the end of each cycle, the four-carbon oxaloacetate has been regenerated, and the cycle continues.
Steps
There are ten basic steps in the citric acid cycle, as outlined below. The cycle is continuously supplied with new carbon in the form of acetyl-CoA, entering at step 0 in the table.[14]
| Reaction type | Substrates | Enzyme | Products | Comment | |
|---|---|---|---|---|---|
| 0 / 10 | Aldol condensation | Acetyl CoA + H2O
|
Citrate synthase | Citrate + CoA-SH | irreversible, extends the 4C oxaloacetate to a 6C molecule |
| 1 | Dehydration | Citrate
|
Aconitase | cis-Aconitate + H2O | reversible isomerisation |
| 2 | Hydration | cis-Aconitate + H2O
|
Isocitrate | ||
| 3 | Oxidation
|
Isocitrate + NAD +
|
Isocitrate dehydrogenase | Oxalosuccinate + NADH + H + | generates NADH (equivalent of 2.5 ATP)
|
| 4 | Decarboxylation | Oxalosuccinate
|
α-Ketoglutarate + CO2
|
rate-limiting, irreversible stage, generates a 5C molecule | |
| 5 | Oxidative decarboxylation |
α-Ketoglutarate + NAD+ + CoA-SH
|
, Mg++,transsuccinytase | Succinyl-CoA + NADH + H + + CO2 | irreversible stage, generates NADH (equivalent of 2.5 ATP), regenerates the 4C chain (CoA excluded) |
| 6 | substrate-level phosphorylation |
Pi
|
Succinyl-CoA synthetase | Succinate + CoA-SH + GTP | or Pi and hydrolysis of succinyl-CoA involve the H2O needed for balanced equation.
|
| 7 | Oxidation | ubiquinone (Q)
|
Succinate dehydrogenase | Fumarate + ubiquinol (QH2) | uses FAD as a prosthetic group (FAD→FADH2 in the first step of the reaction) in the enzyme.[15] These two electrons are later transferred to QH2 during Complex II of the ETC, where they generate the equivalent of 1.5 ATP |
| 8 | Hydration | Fumarate + H2O
|
Fumarase | L-Malate | Hydration of C-C double bond |
| 9 | Oxidation | L-Malate + NAD+
|
Malate dehydrogenase | Oxaloacetate + NADH + H+
|
reversible (in fact, equilibrium favors malate), generates NADH (equivalent of 2.5 ATP)
|
| 10 / 0 | Aldol condensation | Acetyl CoA + H2O
|
Citrate synthase | Citrate + CoA-SH | This is the same as step 0 and restarts the cycle. The reaction is irreversible and extends the 4C oxaloacetate to a 6C molecule |
Two
Mitochondria in animals, including humans, possess two succinyl-CoA synthetases: one that produces GTP from GDP, and another that produces ATP from ADP.[16] Plants have the type that produces ATP (ADP-forming succinyl-CoA synthetase).[14] Several of the enzymes in the cycle may be loosely associated in a multienzyme protein complex within the mitochondrial matrix.[17]
The GTP that is formed by GDP-forming succinyl-CoA synthetase may be utilized by nucleoside-diphosphate kinase to form ATP (the catalyzed reaction is GTP + ADP → GDP + ATP).[15]
Products
Products of the first turn of the cycle are one GTP (or ATP), three NADH, one FADH2 and two CO2.
Because two acetyl-CoA
| Description | Reactants | Products |
|---|---|---|
| The sum of all reactions in the citric acid cycle is: | Acetyl-CoA + 3 NAD+ + FAD + GDP + Pi + 2 H2O | → CoA-SH + 3 NADH + FADH2 + 3 H+ + GTP + 2 CO2 |
| Combining the reactions occurring during the pyruvate oxidation with those occurring during the citric acid cycle, the following overall pyruvate oxidation reaction is obtained: | Pyruvate ion + 4 NAD+ + FAD + GDP + Pi + 2 H2O | → 4 NADH + FADH2 + 4 H+ + GTP + 3 CO2 |
| Combining the above reaction with the ones occurring in the course of glycolysis, the following overall glucose oxidation reaction (excluding reactions in the respiratory chain) is obtained: | Glucose + 10 NAD+ + 2 FAD + 2 ADP + 2 GDP + 4 Pi + 2 H2O | → 10 NADH + 2 FADH2 + 10 H+ + 2 ATP + 2 GTP + 6 CO2 |
The above reactions are balanced if Pi represents the H2PO4− ion, ADP and GDP the ADP2− and GDP2− ions, respectively, and ATP and GTP the ATP3− and GTP3− ions, respectively.
The total number of ATP molecules obtained after complete oxidation of one glucose in glycolysis, citric acid cycle, and oxidative phosphorylation is estimated to be between 30 and 38.[19]
Efficiency
The theoretical maximum yield of
Variation
While the citric acid cycle is in general highly conserved, there is significant variability in the enzymes found in different taxa[22] (note that the diagrams on this page are specific to the mammalian pathway variant).
Some differences exist between eukaryotes and prokaryotes. The conversion of D-threo-isocitrate to 2-oxoglutarate is catalyzed in eukaryotes by the NAD+-dependent EC 1.1.1.41, while prokaryotes employ the NADP+-dependent EC 1.1.1.42.[23] Similarly, the conversion of (S)-malate to oxaloacetate is catalyzed in eukaryotes by the NAD+-dependent EC 1.1.1.37, while most prokaryotes utilize a quinone-dependent enzyme, EC 1.1.5.4.[24]
A step with significant variability is the conversion of succinyl-CoA to succinate. Most organisms utilize EC 6.2.1.5, succinate–CoA ligase (ADP-forming) (despite its name, the enzyme operates in the pathway in the direction of ATP formation). In mammals a GTP-forming enzyme, succinate–CoA ligase (GDP-forming) (EC 6.2.1.4) also operates. The level of utilization of each isoform is tissue dependent.[25] In some acetate-producing bacteria, such as Acetobacter aceti, an entirely different enzyme catalyzes this conversion – EC 2.8.3.18, succinyl-CoA:acetate CoA-transferase. This specialized enzyme links the TCA cycle with acetate metabolism in these organisms.[26] Some bacteria, such as Helicobacter pylori, employ yet another enzyme for this conversion – succinyl-CoA:acetoacetate CoA-transferase (EC 2.8.3.5).[27]
Some variability also exists at the previous step – the conversion of 2-oxoglutarate to succinyl-CoA. While most organisms utilize the ubiquitous NAD+-dependent 2-oxoglutarate dehydrogenase, some bacteria utilize a ferredoxin-dependent 2-oxoglutarate synthase (EC 1.2.7.3).[28] Other organisms, including obligately autotrophic and methanotrophic bacteria and archaea, bypass succinyl-CoA entirely, and convert 2-oxoglutarate to succinate via
In
Regulation
Allosteric regulation by metabolites. The regulation of the citric acid cycle is largely determined by product inhibition and substrate availability. If the cycle were permitted to run unchecked, large amounts of
Citrate is used for feedback inhibition, as it inhibits phosphofructokinase, an enzyme involved in glycolysis that catalyses formation of fructose 1,6-bisphosphate, a precursor of pyruvate. This prevents a constant high rate of flux when there is an accumulation of citrate and a decrease in substrate for the enzyme.[34]
Regulation by calcium. Calcium is also used as a regulator in the citric acid cycle. Calcium levels in the mitochondrial matrix can reach up to the tens of micromolar levels during cellular activation.
Transcriptional regulation. There is a link between intermediates of the citric acid cycle and the regulation of
Major metabolic pathways converging on the citric acid cycle
Several
In this section and in the next, the citric acid cycle intermediates are indicated in italics to distinguish them from other substrates and end-products.
However, it is also possible for pyruvate to be
In the citric acid cycle all the intermediates (e.g.
In the liver, the carboxylation of
In
In
In many tissues, especially heart and skeletal
The total energy gained from the complete breakdown of one (six-carbon) molecule of glucose by glycolysis, the formation of 2 acetyl-CoA molecules, their catabolism in the citric acid cycle, and oxidative phosphorylation equals about 30 ATP molecules, in eukaryotes. The number of ATP molecules derived from the beta oxidation of a 6 carbon segment of a fatty acid chain, and the subsequent oxidation of the resulting 3 molecules of acetyl-CoA is 40.[citation needed]
Citric acid cycle intermediates serve as substrates for biosynthetic processes
In this subheading, as in the previous one, the TCA intermediates are identified by italics.
Several of the citric acid cycle intermediates are used for the synthesis of important compounds, which will have significant cataplerotic effects on the cycle.[40] Acetyl-CoA cannot be transported out of the mitochondrion. To obtain cytosolic acetyl-CoA, citrate is removed from the citric acid cycle and carried across the inner mitochondrial membrane into the cytosol. There it is cleaved by
The carbon skeletons of many
Of these amino acids, aspartate and glutamine are used, together with carbon and nitrogen atoms from other sources, to form the
The
The majority of the carbon atoms in the porphyrins come from the citric acid cycle intermediate, succinyl-CoA. These molecules are an important component of the hemoproteins, such as hemoglobin, myoglobin and various cytochromes.[40]
During gluconeogenesis
Because the citric acid cycle is involved in both
- ^ The interactive pathway map can be edited at WikiPathways: "TCACycle_WP78".
Glucose feeds the TCA cycle via circulating lactate
The metabolic role of lactate is well recognized as a fuel for tissues, mitochondrial cytopathies such as DPH Cytopathy, and the scientific field of oncology (tumors). In the classical Cori cycle, muscles produce lactate which is then taken up by the liver for gluconeogenesis. New studies suggest that lactate can be used as a source of carbon for the TCA cycle.[45]
Evolution
It is believed that components of the citric acid cycle were derived from
See also
References
- ISBN 978-0-12-181870-8.
- ISBN 978-0-904498-22-6.
- ISBN 978-1-59184-646-8.
- ISBN 978-0-393-06596-1.
- PMID 23378250.
- ^ a b c Voet D, Voet JG (2004). Biochemistry (3rd ed.). New York: John Wiley & Sons, Inc. p. 615.
- OCLC 769803483.
- ^ "The Nobel Prize in Physiology or Medicine 1937". The Nobel Foundation. Retrieved 2011-10-26.
- ^ Chandramana, Sudeep. (2014). Inclusive Growth And Youth Empowerment: A Development Model For Aspirational India. Journal of Science, Technology and Management. 7. 52–62.
- PMID 16746382.
- ^ "The Nobel Prize in Physiology or Medicine 1953". The Nobel Foundation. Retrieved 2011-10-26.
- PMID 2106256.
- ISBN 0-7167-3051-0.
- ^ ISBN 978-0-943088-39-6.
- ^ ISBN 978-0-7167-4684-3.
- PMID 9765291.
- S2CID 43052163.
- ^ "The citric acid cycle". Khan Academy. Retrieved 10 August 2021.
- ^ PMID 7654171.
- ISBN 978-0-7167-4684-3.
- S2CID 32361233.
- ^ "Citric acid cycle variants at MetaCyc".
- S2CID 12950388.
- PMID 11092847.
- PMID 15234968.
- PMID 18502856.
- PMID 9325289.
- PMID 19936047.
- S2CID 206536295.
- PMID 28375741.
- PMID 31636445.
- PMID 26629338.
- PMID 21941621.
- ISBN 978-1-319-22800-2.
- PMID 23746507.
- S2CID 27367543.
- PMID 17182618.
- PMID 12087111.
- ^ ISBN 978-0-471-21495-3.
- ^ ISBN 978-0-7167-2009-6.
- OCLC 777722371.
- PMID 12231658.
- PMID 2647392.
- PMID 17344645.
this process is outlined graphically in page 73
- PMID 29045397.
- PMID 3332996.
- PMID 28584880. 83.
- (PDF) from the original on 2017-08-12.
- (PDF) from the original on 2003-05-08.