Dronedarone
 | |
| Clinical data | |
|---|---|
| Trade names | Multaq |
| Other names | SR33589 |
| AHFS/Drugs.com | Monograph |
| MedlinePlus | a609034 |
| License data |
|
| Pregnancy category |
|
| Routes of administration | By mouth |
| ATC code | |
| Legal status | |
| Legal status | |
| Pharmacokinetic data | |
| Bioavailability | 15% (with a high-fat meal)[2] |
| Protein binding | >98% |
| Metabolism | Liver (mainly by CYP3A) |
| Elimination half-life | 13–19 hours |
| Excretion | Feces (84%), urine (~6%) |
| Identifiers | |
| |
JSmol) | |
| |
| |
| | |
Dronedarone, sold under the brand name Multaq, is a class III antiarrhythmic
The FDA label for dronedarone includes a
It is approved as a
Mechanism of action
Dronedarone has been termed a "multichannel blocker".[
Chemistry
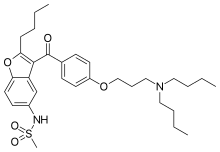
Chemically, dronedarone is a benzofuran derivative related to amiodarone, a popular antiarrhythmic.[medical citation needed] The use of amiodarone is limited by toxicity due its high iodine content (pulmonary fibrosis, thyroid disease) as well as by liver disease.[medical citation needed] In dronedarone, the iodine moieties are not present, reducing toxic effects on the thyroid and other organs.[medical citation needed] A methylsulfonamide group is added to reduce solubility in fats (lipophobicity) and thus reduce neurotoxic effects.[4]
Dronedarone displays amiodarone-like
Pharmacokinetics
Dronedarone is less lipophilic than amiodarone, has a much smaller volume of distribution, and has an elimination half-life of 13–19 hours—this stands in contrast to amiodarone's half-life of several weeks.[2][13] As a result of these pharmacokinetic characteristics, dronedarone dosing may be less complicated than amiodarone.[medical citation needed]
Contraindications
- Permanent AF (patients in whom normal sinus rhythm will not or cannot be restored)[2]
- Recently decompensated heart failure requiring hospitalization or Class IV heart failure.[2]
- Second-or third-degree AV block or sick sinus syndrome (except when used in conjunction with a functioning pacemaker)[2]
- Bradycardia[2]
- Concomitant use of a strong CYP3A inhibitor[2]
- Concomitant use of drugs or herbal products that prolong the QT interval and may induce Torsade de Pointes[2]
- Liver or lung toxicity related to the previous use of amiodarone[2]
- Severe hepatic impairment[2]
- QTc Bazett interval ≥500 ms,[2] or use with drugs or herbal supplements that prolong QT interval or increase risk of torsades de points (Class I or III antiarrhythmic agents, phenothiazines, tricyclic antidepressants, certain oral macrolides, ephedra).[citation needed]
- Pregnancy and nursing mothers[2]
- Hypersensitivity to dronedarone[2]
- Hepatic impairment. In January 2011, the FDA advised about cases of rare, but severe, liver injury, including two cases of acute liver failure leading to liver transplant in patients treated with dronedarone (Multaq). It is not known whether routine periodic monitoring of serum liver enzymes (ALT, AST, and alkaline phosphatase) and bilirubin in patients taking dronedarone will prevent the development of severe liver injury.[6]
- PR interval exceeding 280 ms [citation needed]
- Use of cytochrome P-450 (CYP) 3a isoenzyme inhibitors (includes: clarithromycin, cyclosporine, itraconazole, ketoconazole, nefazodone, ritonavir, telithromycin, voriconazole)
Clinical trials
Clinical trials have compared dronedarone to placebo and to amiodarone, for its ability to reduce atrial fibrillation, to reduce mortality overall and from cardiac causes, and for its adverse effects, including excess mortality.
In the EURIDIS and ADONIS trials in atrial fibrillation (2007), dronedarone was significantly more effective than placebo in maintaining sinus rhythm, with no difference in lung and thyroid function in the short term.[15]
However, in the ANDROMEDA study (2007), dronedarone doubled the death rate compared to placebo, and the trial was halted early.[5] ANDROMEDA enrolled patients with moderate to severe congestive heart failure, a relatively sicker patient population.[medical citation needed]
In a later atrial fibrillation trial, ATHENA, with 4628 subjects, dronedarone was significantly more effective than placebo in reducing the composite endpoint of first hospitalization due to cardiovascular events or death.[16] There was a significant reduction in the rate of cardiovascular death, but not in the rate of death from any cause.[4] Later post-hoc analysis of the ATHENA-results showed a significant reduction in the rate of stroke.[14]
Patients
The PALLAS trial (2011) was stopped for safety concerns due to the finding that "dronedarone increased rates of heart failure, stroke, and death from cardiovascular causes in patients with permanent atrial fibrillation who were at risk for major vascular events".[17] A Black Box warning was subsequently added by the FDA stating that the risk of death, stroke, and hospitalization for congestive heart failure doubled in patients with permanent atrial fibrillation.[medical citation needed]
Direct current cardioversion results
Dronedarone has been tested in some trials as a way to improve the success rate of electrical cardioversion.[medical citation needed] In one such trial by the Veteran's Administration it was used prepare patients for electrical conversion to sinus rhythm.[medical citation needed] In the ATHENA study, 25% of patients were started on dronedarone before cardioversion.[16] The results of a recently concluded randomized study (ELECTRA) may clarify the safety and ideal modalities of dronedarone use at the time of cardioversion.[18]
Regulatory review
Originally submitted as a New Drug Application in 2005, dronedarone was reviewed and recommended for approval in March 2009, by an Advisory Committee of the United States Food and Drug Administration (FDA).[19] The FDA approved dronedarone in July 2009.[citation needed]
Health Canada was the second major regulatory body to approve the drug, giving its approval in August 2009.[citation needed] The approval is for "treatment of patients with a history of, or current atrial fibrillation to reduce their risk of cardiovascular hospitalization due to this condition."[20]
The European Medicines Agency issued a Summary of Positive Opinion regarding dronedarone in September 2009 recommending to the European Commission to grant a marketing authorization within the European Union.[21]
Research
In July 2019, a new drug called
References
- FDA. Retrieved October 22, 2023.
- ^ a b c d e f g h i j k l m n o "Multaq- dronedarone tablet, film coated". DailyMed. October 15, 2020. Retrieved November 18, 2020.
- ^ "FDA Approves Multaq to Treat Heart Rhythm Disorder" (Press release). U.S. Food and Drug Administration (FDA). July 2, 2009. Archived from the original on July 4, 2009. Retrieved July 2, 2009.
- ^ PMID 19403901.
- ^ PMID 18565860.
- ^ a b "FDA Drug Safety Communication: Severe liver injury associated with the use of dronedarone (marketed as Multaq). Safety Announcement". U.S. Food and Drug Administration (FDA). January 14, 2011.
- ^ "First-Time Generic Drug Approvals 2024". U.S. Food and Drug Administration (FDA). March 8, 2024. Retrieved March 9, 2024.
- ^ PMID 11117382.
- ^ PMID 10604978.
- PMID 17804843.
- PMID 10578003.
- ^ "Medscape Drugs & Diseases - Comprehensive peer-reviewed medical condition, surgery, and clinical procedure articles with symptoms, diagnosis, staging, treatment, drugs and medications, prognosis, follow-up, and pictures".
- S2CID 22339555.
- ^ PMID 19752319.
- PMID 17804843.
- ^ PMID 19213680.
- PMID 22082198.
- ^ Clinical trial number NCT01026090 for "A Phase IV, Double-blind, Placebo-controlled, Canadian Multicentre Study Comparing Two Treatment Strategies of Dronedarone Administration Following ELECTive caRdioversion for Prevention of Symptomatic Atrial Fibrillation (AF) Recurrence" at ClinicalTrials.gov
- ^ "FDA briefing document on dronedarone" (PDF). Food and Drug Administration. Archived from the original (PDF) on March 3, 2017. Retrieved December 16, 2019.
- ^ "Multaq® (Dronedarone) for Atrial Fibrillation Now Approved in Canada - insciences". Archived from the original on July 18, 2011. Retrieved August 13, 2009.
- ^ "Summary of Positive Opinion for Multaq" (PDF). European Medicines Agency. September 24, 2009. Retrieved December 1, 2009.
- ^ US20220267288A1, Chan, Chun Yong Eric; Karkhanis, Aneesh Vidyadhar & Venkatesan, Gopalakrishnan, "Poyendarone, a cardiac therapeutic", issued 2022-08-25
- PMID 36213535.
- ^ "New drug molecule for treatment of atrial fibrillation". Medicalxpress. July 18, 2022.
