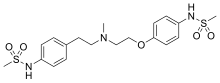
Dofetilide
 | |
| Clinical data | |
|---|---|
| Trade names | Tikosyn |
| AHFS/Drugs.com | Monograph |
| MedlinePlus | a601235 |
| ATC code | |
| Legal status | |
| Legal status | |
| Pharmacokinetic data | |
| Bioavailability | 96% (oral) |
| Protein binding | 60% -70% |
| Elimination half-life | 10 hours |
| Identifiers | |
| |
JSmol) | |
| |
| |
| (verify) | |
Dofetilide is a class III
Medical uses
Dofetilide is used for the maintenance of sinus rhythm in individuals prone to the occurrence of atrial fibrillation and flutter arrhythmias, and for chemical cardioversion to sinus rhythm from atrial fibrillation and flutter.[5][6]
Based on the results of the Danish Investigations of Arrhythmias and Mortality on Dofetilide ("DIAMOND") study,
It has clinical advantages over other class III antiarrhythmics in chemical cardioversion of atrial fibrillation, and maintenance of sinus rhythm, and does not have the pulmonary or hepatotoxicity of amiodarone, however atrial fibrillation is not generally considered life-threatening, and dofetilide causes an increased rate of potentially life-threatening arrhythmias in comparison to other therapies.[8]
Contraindications
Prior to administration of the first dose, the
If at any time after the second dose of dofetilide the QTc is greater than 500 msec (550 msec in patients with ventricular conduction abnormalities), dofetilide should be discontinued. [9]
Adverse effects
Torsades de pointes is the most serious side effect of dofetilide therapy. The incidence of torsades de pointes is 0.3-10.5% and is dose-related, with increased incidence associated with higher doses. The majority of episodes of torsades de pointes have occurred within the first three days of initial dosing. Patients should be hospitalized and monitored for the first three days after starting dofetilide.[7]
The risk of inducing torsades de pointes can be decreased by taking precautions when initiating therapy, such as hospitalizing individuals for a minimum of three days for serial creatinine measurement, continuous telemetry monitoring and availability of cardiac resuscitation.
Pharmacology
Mechanism of action
Dofetilide works by selectively blocking the rapid component of the
This causes the refractory period of atrial tissue to increase, hence its effectiveness in the treatment of atrial fibrillation and atrial flutter.
Dofetilide does not affect dV/dTmax (the slope of the upstroke of phase 0 depolarization), conduction velocity, or the resting membrane potential.

There is a dose-dependent increase in the QT interval and the corrected QT interval (QTc). Because of this, many practitioners will initiate dofetilide therapy only on individuals under telemetry monitoring or if serial EKG measurements of QT and QTc can be performed.
Pharmacokinetics
Peak plasma concentrations are seen two to three hours after oral dosing when fasting. Dofetilide is well absorbed in its oral form, with a bioavailability of >90%. Intravenous administration of dofetilide is not available in the United States.[11]
The elimination
Dofetilide is metabolized predominantly by
Metabolism
A steady-state plasma level of dofetilide is achieved in 2–3 days.
80% of dofetilide is excreted by the
In the kidneys, dofetilide is eliminated via cation exchange (secretion). Agents that interfere with the renal cation exchange system, such as verapamil, cimetidine, hydrochlorothiazide, itraconazole, ketoconazole, prochlorperazine, and trimethoprim should not be administered to individuals taking dofetilide.
About 20 percent of dofetilide is metabolized in the liver via the CYP3A4 isoenzyme of the cytochrome P450 enzyme system. Drugs that interfere with the activity of the CYP3A4 isoenzyme can increase serum dofetilide levels. If the renal cation exchange system is interfered with (as with the medications listed above), a larger percentage of dofetilide is cleared via the CYP3A4 isoenzyme system.
History
After its initial US FDA approval, due to the pro-arrhythmic potential, it was only made available to hospitals and prescribers that had received education and undergone specific training in the risks of treatment with dofetilide; however, this restriction was subsequently removed in 2016. [14]
See also
- Antiarrhythmic agents
- Cardiac action potential
- Electrocardiogram
References
- FDA. Retrieved 22 Oct 2023.
- S2CID 19897963.
- ^ a b Wathion N (2004-04-13). "Public Statement on Tikosyn (dofetilide): Voluntary Withdrawal of the Marketing Authorisation in the European Union" (PDF). European Agency for the Evaluation of Medicinal Products. Archived from the original (PDF) on 2017-10-13. Retrieved 2015-01-03.
- ^ Australian Medicines Handbook 2014
- S2CID 25162347.
- PMID 12845335.
- ^ PMID 10486417.
- ^ Micromedex Drugdex drug evaluations micromedex.com
- ^ "TIKOSYN® (dofetilide)". Pfizer.
- S2CID 11255636.
- PMID 1279316.
- ^ "Dofetilide." Lexicomp. Wulters Kluwer Health, n.d. Web. <online.lexi.com>.
- PMID 8801060.
- ^ "Information for Tikosyn (dofetilide)". US Food and Drug Administration. 2016-03-09.
