Osmium compounds
| Oxidation states of osmium | |
|---|---|
| −2 | Na 2[Os(CO) 4] |
| −1 | Na 2[Os 4(CO) 13] |
| 0 | Os 3(CO) 12 |
| +1 | OsI |
| +2 | OsI 2 |
| +3 | OsBr 3 |
| +4 | OsO 2, OsCl 4 |
| +5 | OsF 5 |
| +6 | OsF 6 |
| +7 | OsOF 5 |
| +8 | OsO 4, Os(NCH 3) 4 |
Osmium compounds are compounds containing the element
Oxides

Osmium tetroxide is the most notable compound of osmium, having many uses. The name "osmium" even derives from Greek "ὀσμή, osme, 'smell'" because of the smell of osmium tetroxide.[12] It also has a number of unusual properties, one being that the solid is volatile. Its volatility, along with its strong oxidizing power, is the origin of its quite serious toxicity - inhalation provides a very effective route for the compound to react with tissue. The compound is colourless, but most samples appear yellow.[13] This is most likely due to the presence of the impurity OsO2, which is yellow-brown in colour.[14] In biology, its property of binding to lipids has made it a widely used stain in electron microscopy. OsO4 is formed slowly when osmium powder reacts with O2 at ambient temperature. Reaction of bulk solid requires heating to 400 °C.[15]
OsO4 is a
- OsO4 + Me3CNH2 → OsO3(NCMe3) + H2O
Similarly, with
- OsO4 + NH3 + KOH → K[Os(N)O3] + 2 H2O
The [Os(N)O3]− anion is isoelectronic and isostructural with OsO4. OsO4 is very soluble in
- OsO4 + 4 H2 → Os + 4 H2O
OsO4 undergoes "reductive carbonylation" with carbon monoxide in methanol at 400 K and 200 sbar to produce the triangular cluster Os3(CO)12:
- 3 OsO4 + 24 CO → Os3(CO)12 + 12 CO2[15]

Osmium dioxide is another known oxide of osmium, which can be obtained by the reaction of osmium with a variety of oxidizing agents, including, sodium chlorate, osmium tetroxide, and nitric oxide at about 600 °C.[18][19] It does not dissolve in water, but is attacked by dilute hydrochloric acid.[20][21] The crystals have rutile structure.[22] Unlike osmium tetroxide, OsO2 is not toxic.[23]
Halides
Fluorides

Osmium hexafluoride is one of the 17 known binary hexafluorides, which can be made by the direct reaction of osmium metal exposed to an excess of elemental fluorine gas at 300 °C. It is a yellow crystalline solid that melts at 33.4 °C and boils at 47.5 °C.[24] The solid structure measured at −140 °C is orthorhombic space group Pnma. Lattice parameters are a = 9.387 Å, b = 8.543 Å, and c = 4.944 Å. There are four formula units (in this case, discrete molecules) per unit cell, giving a density of 5.09 g·cm−3.[25] The OsF6 molecule itself (the form important for the liquid or gas phase) has octahedral molecular geometry, which has point group (Oh). The Os–F bond length is 1.827 Å.[25] Partial hydrolysis of OsF6 produces OsOF4.[26] Osmium pentafluoride is a tetramer in the solid state that can be prepared by reduction of osmium hexafluoride with iodine as a solution in iodine pentafluoride:[27]
- 10 OsF6 + I2 → 10 OsF5 + 2 IF5
Chlorides
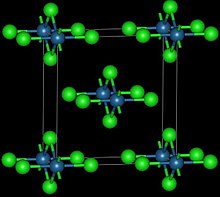
- Os + 2 Cl2 → OsCl4
This reddish-black polymorph is orthorhombic and adopts a structure in which osmium centres are octahedrally coordinated, sharing opposite edges of the OsCl6 octahedra to form a chain.[30] A brown, apparently cubic polymorph forms upon reduction of osmium tetroxide with thionyl chloride:[31]
- OsO4 + 4 SOCl2 → OsCl4 + 2 Cl2 + 4 SO2
Osmium tetroxide dissolves in hydrochloric acid to give the hexachloroosmate anion:
- OsO4 + 10 HCl → H2OsCl6 + 2 Cl2 + 4 H2O
Bromides

Iodides
Osmium(I) iodide is a metallic grey solid produced by the reaction of
- OsO4 + HI → OsI2 + H2O
This compound decomposes in contact with water.
Borides

See also
References
- ^ Stoye, Emma (23 October 2014). "Iridium forms compound in +9 oxidation state". Chemistry World. Royal Society of Chemistry.
- S2CID 29205117.
- S2CID 28547895.
- .
- ^ "Chemistry of Hassium" (PDF). Gesellschaft für Schwerionenforschung mbH. 2002. Archived from the original (PDF) on 2012-01-14. Retrieved 2007-01-31.
- ]
- S2CID 100915090.
- PMID 23883027.
- .
- .
- .
- OCLC 23991202.
- PMID 23089872.
- ^ Cotton and Wilkinson, Advanced Inorganic Chemistry, p.1002
- ^ ISBN 978-0-13-039913-7.
- ISBN 978-0-08-037941-8.
- ^ Kiernan, J. A. "Re: "Disposal" of Osmium Tetroxide "Waste"". Department of Anatomy & Cell Biology, The University of Western Ontario.
- ISBN 0-12-352651-5.
- .
- .
- .
- .
- PMID 4470919.)
{{cite journal}}: CS1 maint: multiple names: authors list (link - ISBN 978-1-4200-9084-0, Section 4, Physical Constants of Inorganic Compounds, p. 4-85.
- ^ PMID 16634614.
- .
- .
- .
- ISBN 0-7514-0413-6.
- ISBN 0-19-855370-6.
- .
- .
- ISBN 978-1-119-95143-8.
- ^ .
- ^ ISBN 978-0-19-974219-6. Retrieved 11 November 2021.
- .
- ^ PMID 15898746.
- .
- .
- .
- .

![{\displaystyle {\ce {Os + 2O2 ->[\Delta T] OsO4}}}](https://wikimedia.org/api/rest_v1/media/math/render/svg/8d2c2ac06e64cd164869ed5506f13c3cfa320d70)