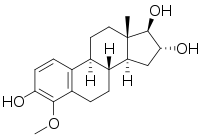
4-Methoxyestriol
| |
| Names | |
|---|---|
| IUPAC name
4-Methoxyestra-1,3,5(10)-triene-3,16α,17β-triol
| |
| Systematic IUPAC name
(1R,2R,3aS,3bR,9bS,11aS)-6-Methoxy-11a-methyl-2,3,3a,3b,4,5,9b,10,11,11a-decahydro-1H-cyclopenta[a]phenanthrene-1,2,7-triol | |
| Other names
4-MeO-E3
| |
| Identifiers | |
3D model (
JSmol ) |
|
| ChemSpider | |
PubChem CID
|
|
| |
| |
| Properties | |
| C19H26O4 | |
| Molar mass | 318.413 g·mol−1 |
Except where otherwise noted, data are given for materials in their standard state (at 25 °C [77 °F], 100 kPa).
| |
4-Methoxyestriol (4-MeO-E3) is an
relative binding affinities (RBAs) for estrogen receptor alpha (ERα) and estrogen receptor beta (ERβ) are both about 1% of those of estradiol.[4] For comparison, estriol had RBAs of 11% and 35%, respectively.[4]
See also
References
