Hofmann elimination
| Hofmann elimination | |
|---|---|
| Named after | August Wilhelm von Hofmann |
| Reaction type | Elimination reaction |
| Identifiers | |
| Organic Chemistry Portal | hofmann-elimination |
| RSC ontology ID | RXNO:0000166 |
Hofmann elimination is an
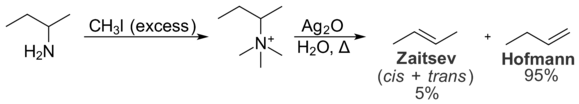
The reaction starts with the formation of a
In the Hofmann elimination, the least substituted alkene is typically favored due to intramolecular steric interactions. The quaternary ammonium group is large, and interactions with alkyl groups on the rest of the molecule are undesirable. As a result, the conformation necessary for the formation of the Zaitsev product is less energetically favorable than the conformation required for the formation of the Hofmann product. As a result, the Hofmann product is formed preferentially. The
An example of a Hofmann elimination (not involving a contrast between a Zaitsev product and a Hofmann product) is the synthesis of
In a related chemical test, known as the Herzig–Meyer alkimide group determination, a tertiary amine with at least one methyl group and lacking a beta-proton is allowed to react with hydrogen iodide to the quaternary ammonium salt which when heated degrades to methyl iodide and the secondary amine.[5]
See also
- Cope elimination
- Emde degradation
References
- S2CID 108453887.
- .
- ^ Wade, p. 903.
- ^ Arthur C. Cope; Robert D. Bach (1973). "trans-Cyclooctene". Organic Syntheses; Collected Volumes, vol. 5, p. 315.
- .