Microglia
| Microglia | |
|---|---|
 Microglia in resting state from rat cortex before traumatic brain injury (lectin staining with HRP) | |
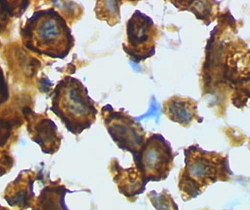 Microglia/macrophage – activated form from rat cortex after traumatic brain injury (lectin staining with HRP) | |
| Details | |
| Precursor | Primitive yolk-sac derived macrophage |
| System | Central nervous system |
| Identifiers | |
| MeSH | D017628 |
| TH | H2.00.06.2.00004, H2.00.06.2.01025 |
| FMA | 54539 |
| Anatomical terms of microanatomy | |
Microglia are a type of
The brain and spinal cord, which make up the CNS, are not usually accessed directly by pathogenic factors in the body's circulation due to a series of
History
The ability to view and characterize different neural cells including microglia began in 1880 when Nissl staining was developed by
]Forms
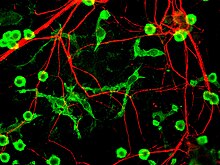
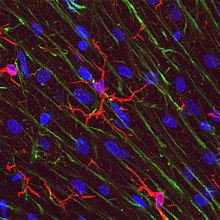
Microglial cells are extremely
Ramified
This form of microglial cell is commonly found at specific locations throughout the entire brain and spinal cord in the absence of foreign material or dying cells. This "resting" form of microglia is composed of long branching processes and a small cellular body. Unlike the amoeboid forms of microglia, the cell body of the ramified form remains in place while its branches are constantly moving and surveying the surrounding area. The branches are very sensitive to small changes in physiological condition and require very specific culture conditions to observe in vitro.[15]
Unlike activated or ameboid microglia, ramified microglia do not phagocytose cells and secrete fewer immunomolecules (including the MHC class I/II proteins). Microglia in this state are able to search for and identify immune threats while maintaining homeostasis in the CNS.[16][17][18] Although this is considered the resting state, microglia in this form are still extremely active in chemically surveying the environment. Ramified microglia can be transformed into the activated form at any time in response to injury or threat.[15]
Reactive (Activated)
Although historically frequently used, the term "activated" microglia should be replaced by "reactive" microglia.
Non-phagocytic
This state is actually part of a graded response as microglia move from their ramified form to their fully active phagocytic form. Microglia can be activated by a variety of factors including: pro-inflammatory
Phagocytic
Activated phagocytic microglia are the maximally immune-responsive form of microglia. These cells generally take on a large, ameboid shape, although some variance has been observed. In addition to having the antigen presenting,
Amoeboid
This shape allows the microglia free movement throughout the neural tissue, which allows it to fulfill its role as a scavenger cell. Amoeboid microglia are able to phagocytose debris, but do not fulfill the same antigen-presenting and inflammatory roles as activated microglia. Amoeboid microglia are especially prevalent during the development and rewiring of the brain, when there are large amounts of extracellular debris and apoptotic cells to remove. This form of microglial cell is found mainly within the perinatal white matter areas in the corpus callosum known as the "Fountains of Microglia".[7][17][22]
Gitter cells
Gitter cells are the eventual result of microglial cells' phagocytosis of infectious material or cellular debris. Eventually, after engulfing a certain amount of material, the phagocytic microglial cell becomes unable to phagocytose any further materials. The resulting cellular mass is known as a granular corpuscle, named for its 'grainy' appearance. By looking at tissue stained to reveal gitter cells, pathologists can visualize healed areas post-infection.[23]
Perivascular
Unlike the other types of microglia mentioned above, "perivascular" microglia refers to the location of the cell, rather than its form/function. Perivascular microglia are mainly found encased within the walls of the
Juxtavascular
Like perivascular microglia, juxtavascular microglia can be distinguished mainly by their location. Juxtavascular microglia are found making direct contact with the
Functions

Microglial cells fulfill a variety of different tasks within the CNS mainly related to both immune response and maintaining homeostasis. The following are some of the major known functions carried out by these cells.[citation needed]
Scavenging
In addition to being very sensitive to small changes in their environment, each microglial cell also physically surveys its domain on a regular basis. This action is carried out in the ameboid and resting states. While moving through its set region, if the microglial cell finds any foreign material, damaged cells,
Phagocytosis
The main role of microglia,
Extracellular signaling
A large part of microglial cell's role in the brain is maintaining
Antigen presentation
As mentioned above, resident non-activated microglia act as poor
Cytotoxicity
In addition to being able to destroy infectious organisms through cell to cell contact via
Synaptic stripping
In a phenomenon first noticed in spinal lesions by Blinzinger and Kreutzberg in 1968, post-inflammation microglia remove the branches from nerves near damaged tissue. This helps promote regrowth and remapping of damaged
Promotion of repair
Post-inflammation, microglia undergo several steps to promote regrowth of neural tissue. These include synaptic stripping, secretion of anti-inflammatory
Development

For a long time it was thought that microglial cells differentiate in the bone marrow from hematopoietic stem cells, the progenitors of all blood cells. However, recent studies show that microglia originate in the yolk sac during a remarkably restricted embryonal period and populate the brain parenchyma guided by a precisely orchestrated molecular process.[4] Yolk sac progenitor cells require activation colony stimulating factor 1 receptor (CSF1R) for migration into the brain and differentiation into microglia.[32] Additionally, the greatest contribution to microglial repopulation is based upon its local self-renewal, both in steady state and disease, while circulating monocytes may also contribute to a lesser extent, especially in disease.[4][33]
Monocytes can also differentiate into
In their downregulated form, microglia lack the
Another difference between microglia and other cells that differentiate from myeloid progenitor cells is the turnover rate. Macrophages and
Aging
Microglia undergo a burst of
Accumulation of minor neuronal damage that occurs during normal aging can transform microglia into enlarged and activated cells.
Research has discovered dystrophic (defective development) human microglia. "These cells are characterized by abnormalities in their cytoplasmic structure, such as deramified, atrophic, fragmented or unusually tortuous processes, frequently bearing spheroidal or bulbous swellings."
In mice, it has been shown that CD22 blockade restores homeostatic microglial phagocytosis in aging brains.[38]

Clinical significance

Microglia are the primary immune cells of the central nervous system, similar to peripheral macrophages. They respond to pathogens and injury by changing morphology and migrating to the site of infection/injury, where they destroy pathogens and remove damaged cells. As part of their response they secrete cytokines, chemokines, prostaglandins, and reactive oxygen species, which help to direct the immune response. Additionally, they are instrumental in the resolution of the inflammatory response, through the production of anti-inflammatory cytokines. Microglia have also been extensively studied for their harmful roles in neurodegenerative diseases, such as Alzheimer's disease, Parkinson's disease, Multiple sclerosis, as well as cardiac diseases, glaucoma, and viral and bacterial infections. There is accumulating evidence that immune dysregulation contributes to the pathophysiology of obsessive-compulsive disorder (OCD), Tourette syndrome, and Pediatric Autoimmune Neuropsychiatric Disorders Associated with Streptococcal Infections (PANDAS).[39]
Since microglia rapidly react to even subtle alterations in central nervous system homeostasis, they can be seen as sensors for neurological dysfunctions or disorders.[40] In the event of brain pathologies, the microglial phenotype is certainly altered.[40] Therefore, analyzing microglia can be a sensitive tool to diagnose and characterize central nervous system disorders in any given tissue specimen.[40] In particular, the microglial cell density, cell shape, distribution pattern, distinct microglial phenotypes and interactions with other cell types should be evaluated.[40]
Sensome genetics
The microglial sensome is a relatively new biological concept that appears to be playing a large role in
The regulation of genes within the sensome must be able to change in order to respond to potential harm. Microglia can take on the role of neuroprotection or neurotoxicity in order to face these dangers.[42] For these reasons, it is suspected that the sensome may be playing a role in neurodegeneration. Sensome genes that are upregulated with aging are mostly involved in sensing infectious microbial ligands while those that are downregulated are mostly involved in sensing endogenous ligands.[41] This analysis suggests a glial-specific regulation favoring neuroprotection in natural neurodegeneration. This is in contrast to the shift towards neurotoxicity seen in neurodegenerative diseases.
The sensome can also play a role in neurodevelopment. Early-life brain infection results in microglia that are hypersensitive to later immune stimuli. When exposed to infection, there is an upregulation of sensome genes involved in neuroinflammation and a downregulation of genes that are involved with neuroplasticity.[43] The sensome's ability to alter neurodevelopment may however be able to combat disease. The deletion of CX3CL1, a highly expressed sensome gene, in rodent models of Rett syndrome resulted in improved health and longer lifespan.[44] The downregulation of Cx3cr1 in humans without Rett syndrome is associated with symptoms similar to schizophrenia.[45] This suggests that the sensome not only plays a role in various developmental disorders, but also requires tight regulation in order to maintain a disease-free state.
See also
- Neuroimmune system
- List of human cell types derived from the germ layers
- List of distinct cell types in the adult human body
References
- PMID 23616747.
- PMID 32253358.
- PMID 25110235.
- ^ PMID 36975541.
- PMID 7763326.
- PMID 11756501.
- ^ S2CID 22708728.
- S2CID 36995129.
- ^ Kierdorf and Prinz, J Clin Invest. 2017;127(9): 3201–3209. https://doi.org/10.1172/JCI90602.
- ^ S2CID 209343260.
- ^ Babeş VM (1892). "Certains caractères des lesions histologiques de la rage" [Certain characteristics of the histological lesions of rabies]. Annales de l'Institut Pasteur (in French). 6: 209–23.
- S2CID 3712733.
- ^ del Río Hortega P, Penfield W (1892). "Cerebral Cicatrix: the Reaction of Neuroglia and Microglia to Brain Wounds". Bulletin of the Johns Hopkins Hospital. 41: 278–303.
- ^ del Rio-Hortega F (1937). "Microglia". Cytology and Cellular Pathology of the Nervous System: 481–534.
- ^ ISBN 978-1118402054.[page needed]
- ^ S2CID 25410282.
- ^ S2CID 34491558.
- S2CID 22596219.
- S2CID 15283939.
- PMID 28524175.
- ^ Jelinek HF, Karperien A, Bossomaier T, Buchan A (1975). "Differentiating grades of microglia activation with fractal analysis" (PDF). Complexity International. 12 (18): 1713–7. Archived from the original (PDF) on 2008-12-17.
- ^ S2CID 23457378.
- PMID 32166015.
- ^ PMID 17111048.
- PMID 36994950.
- PMID 26505565.
- PMID 31040847.
- PMID 32483169.
- S2CID 25545853.
- PMID 34738335.
- S2CID 252416407.
- PMID 24890514.
- PMID 26134003.
- S2CID 85495400.
- PMID 8901425.
- ^ S2CID 8874596.
- ^ S2CID 33152515.
- PMID 30944478.
- PMID 28053994.
- ^ license.
- ^ PMID 24162652.
- ^ Block, M.L., Zecca, L. & Hong, J.S. Microglia-mediated neurotoxicity: uncovering the molecular mechanisms. Nat. Rev. Neurosci. 8, 57–69 (2007).
- PMID 26872419.
- PMID 26883520.
- S2CID 205073822.
Further reading
- Rock RB, Gekker G, Hu S, Sheng WS, Cheeran M, Lokensgard JR, Peterson PK (October 2004). "Role of microglia in central nervous system infections". Clinical Microbiology Reviews. 17 (4): 942–64, table of contents. PMID 15489356.
- Han X, Li Q, Lan X, El-Mufti L, Ren H, Wang J (September 2019). "Microglial Depletion with Clodronate Liposomes Increases Proinflammatory Cytokine Levels, Induces Astrocyte Activation, and Damages Blood Vessel Integrity". Molecular Neurobiology. 56 (9): 6184–6196. PMID 30734229.
External links
- Microglia information, videos & resources at microglia.info
- Microglia home page at microglia.net
- Creeping into your Head - A Brief Introduction to Microglia — A Review from the Science Creative Quarterly
- "Immune Scavengers Target Alzheimer's Plaques". April 6, 2007.
- The Department of Neuroscience at Wikiversity
- NIF Search - Microglial Cell via the Neuroscience Information Framework
