Cell nucleus
Animal cell diagram | |
|---|---|
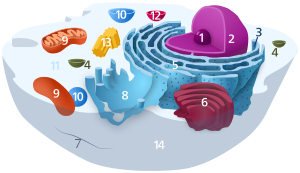 Components of a typical animal cell:
|
The cell nucleus (from
The cell nucleus contains nearly all of the cell's genome. Nuclear DNA is often organized into multiple chromosomes – long strands of DNA dotted with various proteins, such as histones, that protect and organize the DNA. The genes within these chromosomes are structured in such a way to promote cell function. The nucleus maintains the integrity of genes and controls the activities of the cell by regulating gene expression.
Because the nuclear envelope is impermeable to large molecules, nuclear pores are required to regulate nuclear transport of molecules across the envelope. The pores cross both nuclear membranes, providing a channel through which larger molecules must be actively transported by carrier proteins while allowing free movement of small molecules and ions. Movement of large molecules such as proteins and RNA through the pores is required for both gene expression and the maintenance of chromosomes. Although the interior of the nucleus does not contain any membrane-bound subcompartments, a number of nuclear bodies exist, made up of unique proteins, RNA molecules, and particular parts of the chromosomes. The best-known of these is the nucleolus, involved in the assembly of ribosomes.
Structures
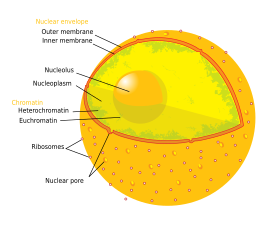
The nucleus contains nearly all of the cell's
Nuclear envelope and pores
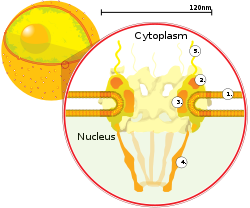
The
In a mammalian nuclear envelope there are between 3000 and 4000
Most proteins, ribosomal subunits, and some RNAs are transported through the pore complexes in a process mediated by a family of transport factors known as karyopherins. Those karyopherins that mediate movement into the nucleus are also called importins, whereas those that mediate movement out of the nucleus are called exportins. Most karyopherins interact directly with their cargo, although some use adaptor proteins.[7] Steroid hormones such as cortisol and aldosterone, as well as other small lipid-soluble molecules involved in intercellular signaling, can diffuse through the cell membrane and into the cytoplasm, where they bind nuclear receptor proteins that are trafficked into the nucleus. There they serve as transcription factors when bound to their ligand; in the absence of a ligand, many such receptors function as histone deacetylases that repress gene expression.[6]: 488
Nuclear lamina
In animal cells, two networks of
The nuclear lamina is composed mostly of
Like the components of other intermediate filaments, the lamin
Mutations in lamin genes leading to defects in filament assembly cause a group of rare genetic disorders known as
Chromosomes
The cell nucleus contains the majority of the cell's genetic material in the form of multiple linear DNA molecules organized into structures called
There are two types of chromatin.
Antibodies to certain types of chromatin organization, in particular,
Nucleolus
The
In the first step of ribosome assembly, a protein called RNA polymerase I transcribes rDNA, which forms a large pre-rRNA precursor. This is cleaved into two large rRNA subunits – 5.8S, and 28S, and a small rRNA subunit 18S.[2]: 328 [24] The transcription, post-transcriptional processing, and assembly of rRNA occurs in the nucleolus, aided by small nucleolar RNA (snoRNA) molecules, some of which are derived from spliced introns from messenger RNAs encoding genes related to ribosomal function. The assembled ribosomal subunits are the largest structures passed through the nuclear pores.[6]: 526
When observed under the
Other nuclear bodies
| Structure name | Structure diameter | Ref. |
|---|---|---|
| Cajal bodies | 0.2–2.0 µm | [25] |
| Clastosomes | 0.2-0.5 µm | [26] |
| PIKA | 5 µm | [27] |
| PML bodies | 0.2–1.0 µm | [28] |
| Paraspeckles | 0.5–1.0 µm | [29] |
| Speckles | 20–25 nm | [27] |
Besides the nucleolus, the nucleus contains a number of other nuclear bodies. These include Cajal bodies, gemini of Cajal bodies, polymorphic interphase karyosomal association (PIKA), promyelocytic leukaemia (PML) bodies, paraspeckles, and splicing speckles. Although little is known about a number of these domains, they are significant in that they show that the nucleoplasm is not a uniform mixture, but rather contains organized functional subdomains.[28]
Other subnuclear structures appear as part of abnormal disease processes. For example, the presence of small intranuclear rods has been reported in some cases of nemaline myopathy. This condition typically results from mutations in actin, and the rods themselves consist of mutant actin as well as other cytoskeletal proteins.[30]
Cajal bodies and gems
A nucleus typically contains between one and ten compact structures called
Similar to Cajal bodies are Gemini of Cajal bodies, or gems, whose name is derived from the
PIKA and PTF domains
PIKA domains, or polymorphic interphase karyosomal associations, were first described in microscopy studies in 1991. Their function remains unclear, though they were not thought to be associated with active DNA replication, transcription, or RNA processing.[34] They have been found to often associate with discrete domains defined by dense localization of the transcription factor PTF, which promotes transcription of small nuclear RNA (snRNA).[35]
PML-nuclear bodies
Promyelocytic leukemia protein (PML-nuclear bodies) are spherical bodies found scattered throughout the nucleoplasm, measuring around 0.1–1.0 µm. They are known by a number of other names, including nuclear domain 10 (ND10), Kremer bodies, and PML oncogenic domains.[36] PML-nuclear bodies are named after one of their major components, the promyelocytic leukemia protein (PML). They are often seen in the nucleus in association with Cajal bodies and cleavage bodies.[28] Pml-/- mice, which are unable to create PML-nuclear bodies, develop normally without obvious ill effects, showing that PML-nuclear bodies are not required for most essential biological processes.[37]
Splicing speckles
Speckles are subnuclear structures that are enriched in pre-messenger RNA splicing factors and are located in the interchromatin regions of the nucleoplasm of mammalian cells.[38] At the fluorescence-microscope level they appear as irregular, punctate structures, which vary in size and shape, and when examined by electron microscopy they are seen as clusters of
Studies on the composition, structure and behaviour of speckles have provided a model for understanding the functional compartmentalization of the nucleus and the organization of the gene-expression machinery[40] splicing snRNPs[41][42] and other splicing proteins necessary for pre-mRNA processing.[40] Because of a cell's changing requirements, the composition and location of these bodies changes according to mRNA transcription and regulation via phosphorylation of specific proteins.[43] The splicing speckles are also known as nuclear speckles (nuclear specks), splicing factor compartments (SF compartments), interchromatin granule clusters (IGCs), and B snurposomes.[44] B snurposomes are found in the amphibian oocyte nuclei and in Drosophila melanogaster embryos. B snurposomes appear alone or attached to the Cajal bodies in the electron micrographs of the amphibian nuclei.[45] IGCs function as storage sites for the splicing factors.[46]
Paraspeckles
Discovered by Fox et al. in 2002, paraspeckles are irregularly shaped compartments in the interchromatin space of the nucleus.[47] First documented in HeLa cells, where there are generally 10–30 per nucleus,[48] paraspeckles are now known to also exist in all human primary cells, transformed cell lines, and tissue sections.[49] Their name is derived from their distribution in the nucleus; the "para" is short for parallel and the "speckles" refers to the splicing speckles to which they are always in close proximity.[48]
Paraspeckles sequester nuclear proteins and RNA and thus appear to function as a molecular sponge
Perichromatin fibrils
Perichromatin fibrils are visible only under electron microscope. They are located next to the transcriptionally active chromatin and are hypothesized to be the sites of active
Clastosomes
Clastosomes are small nuclear bodies (0.2–0.5 µm) described as having a thick ring-shape due to the peripheral capsule around these bodies.[26] This name is derived from the Greek klastos, broken and soma, body.[26] Clastosomes are not typically present in normal cells, making them hard to detect. They form under high proteolytic conditions within the nucleus and degrade once there is a decrease in activity or if cells are treated with proteasome inhibitors.[26][52] The scarcity of clastosomes in cells indicates that they are not required for proteasome function.[53] Osmotic stress has also been shown to cause the formation of clastosomes.[54] These nuclear bodies contain catalytic and regulatory subunits of the proteasome and its substrates, indicating that clastosomes are sites for degrading proteins.[53]
Function
The nucleus provides a site for
Cell compartmentalization
The
In order to control which genes are being transcribed, the cell separates some transcription factor proteins responsible for regulating gene expression from physical access to the DNA until they are activated by other signaling pathways. This prevents even low levels of inappropriate gene expression. For example, in the case of
The compartmentalization allows the cell to prevent translation of unspliced mRNA.[57] Eukaryotic mRNA contains introns that must be removed before being translated to produce functional proteins. The splicing is done inside the nucleus before the mRNA can be accessed by ribosomes for translation. Without the nucleus, ribosomes would translate newly transcribed (unprocessed) mRNA, resulting in malformed and nonfunctional proteins.[6]: 108–15
Replication
The main function of the cell nucleus is to control gene expression and mediate the replication of DNA during the cell cycle.[6]: 171 It has been found that replication happens in a localised way in the cell nucleus. In the S phase of interphase of the cell cycle; replication takes place. Contrary to the traditional view of moving replication forks along stagnant DNA, a concept of replication factories emerged, which means replication forks are concentrated towards some immobilised 'factory' regions through which the template DNA strands pass like conveyor belts.[58]
Gene expression
Gene expression first involves transcription, in which DNA is used as a template to produce RNA. In the case of genes encoding proteins, that RNA produced from this process is messenger RNA (mRNA), which then needs to be translated by ribosomes to form a protein. As ribosomes are located outside the nucleus, mRNA produced needs to be exported.[59]
Since the nucleus is the site of transcription, it also contains a variety of proteins that either directly mediate transcription or are involved in regulating the process. These proteins include
Processing of pre-mRNA
Newly synthesized mRNA molecules are known as
RNA splicing, carried out by a complex called the
Dynamics and regulation
Nuclear transport
The entry and exit of large molecules from the nucleus is tightly controlled by the nuclear pore complexes. Although small molecules can enter the nucleus without regulation,
Nuclear import depends on the importin binding its cargo in the cytoplasm and carrying it through the nuclear pore into the nucleus. Inside the nucleus, RanGTP acts to separate the cargo from the importin, allowing the importin to exit the nucleus and be reused. Nuclear export is similar, as the exportin binds the cargo inside the nucleus in a process facilitated by RanGTP, exits through the nuclear pore, and separates from its cargo in the cytoplasm.[63]
Specialized export proteins exist for translocation of mature mRNA and tRNA to the cytoplasm after post-transcriptional modification is complete. This quality-control mechanism is important due to these molecules' central role in protein translation. Mis-expression of a protein due to incomplete excision of exons or mis-incorporation of amino acids could have negative consequences for the cell; thus, incompletely modified RNA that reaches the cytoplasm is degraded rather than used in translation.[6]
Assembly and disassembly
During its lifetime, a nucleus may be broken down or destroyed, either in the process of cell division or as a consequence of apoptosis (the process of programmed cell death). During these events, the structural components of the nucleus — the envelope and lamina — can be systematically degraded. In most cells, the disassembly of the nuclear envelope marks the end of the
At a certain point during the cell cycle in open mitosis, the cell divides to form two cells. In order for this process to be possible, each of the new daughter cells must have a full set of genes, a process requiring replication of the chromosomes as well as segregation of the separate sets. This occurs by the replicated chromosomes, the
However, in
Apoptosis is a controlled process in which the cell's structural components are destroyed, resulting in death of the cell. Changes associated with apoptosis directly affect the nucleus and its contents, for example, in the condensation of chromatin and the disintegration of the nuclear envelope and lamina. The destruction of the lamin networks is controlled by specialized apoptotic proteases called caspases, which cleave the lamin proteins and, thus, degrade the nucleus' structural integrity. Lamin cleavage is sometimes used as a laboratory indicator of caspase activity in assays for early apoptotic activity.[11] Cells that express mutant caspase-resistant lamins are deficient in nuclear changes related to apoptosis, suggesting that lamins play a role in initiating the events that lead to apoptotic degradation of the nucleus.[11] Inhibition of lamin assembly itself is an inducer of apoptosis.[67]
The nuclear envelope acts as a barrier that prevents both DNA and RNA viruses from entering the nucleus. Some viruses require access to proteins inside the nucleus in order to replicate and/or assemble. DNA viruses, such as
Initially, it has been suspected that
Nuclei per cell
Most
Anucleated cells
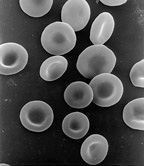
An anucleated cell contains no nucleus and is, therefore, incapable of dividing to produce daughter cells. The best-known anucleated cell is the mammalian red blood cell, or
In flowering plants, this condition occurs in sieve tube elements.[73]
Multinucleated cells
A number of dinoflagellates are known to have two nuclei. Unlike other multinucleated cells these nuclei contain two distinct lineages of DNA: one from the dinoflagellate and the other from a symbiotic diatom.[80]
Evolution
As the major defining characteristic of the eukaryotic cell, the nucleus's evolutionary origin has been the subject of much speculation. Four major hypotheses have been proposed to explain the existence of the nucleus, although none have yet earned widespread support.[81][82][83]
The first model known as the "syntrophic model" proposes that a symbiotic relationship between the archaea and bacteria created the nucleus-containing eukaryotic cell. (Organisms of the Archaea and Bacteria domain have no cell nucleus.[84]) It is hypothesized that the symbiosis originated when ancient archaea, similar to modern methanogenic archaea, invaded and lived within bacteria similar to modern myxobacteria, eventually forming the early nucleus. This theory is analogous to the accepted theory for the origin of eukaryotic mitochondria and chloroplasts, which are thought to have developed from a similar endosymbiotic relationship between proto-eukaryotes and aerobic bacteria.[85] One possibility is that the nuclear membrane arose as a new membrane system following the origin of mitochondria in an archaebacterial host.[86] The nuclear membrane may have served to protect the genome from damaging reactive oxygen species produced by the protomitochondria.[87] The archaeal origin of the nucleus is supported by observations that archaea and eukarya have similar genes for certain proteins, including histones. Observations that myxobacteria are motile, can form multicellular complexes, and possess kinases and G proteins similar to eukarya, support a bacterial origin for the eukaryotic cell.[88]
A second model proposes that proto-eukaryotic cells evolved from bacteria without an endosymbiotic stage. This model is based on the existence of modern
The most controversial model, known as
A more recent proposal, the exomembrane hypothesis, suggests that the nucleus instead originated from a single ancestral cell that evolved a second exterior cell membrane; the interior membrane enclosing the original cell then became the nuclear membrane and evolved increasingly elaborate pore structures for passage of internally synthesized cellular components such as ribosomal subunits.[95]
History


The nucleus was the first organelle to be discovered. What is most likely the oldest preserved drawing dates back to the early microscopist Antonie van Leeuwenhoek (1632–1723). He observed a "lumen", the nucleus, in the red blood cells of salmon.[96] Unlike mammalian red blood cells, those of other vertebrates still contain nuclei.[97]
The nucleus was also described by
In 1838,
Between 1877 and 1878,
See also
References
- PMID 28545058.
- ^ a b c d e f g h i j k Alberts B, Johnson A, Lewis J, Morgan D, Raff M, Roberts K, Walter P (2015). Molecular Biology of the Cell (6 ed.). New York: Garland Science.
- ^ ISBN 978-1-4641-8339-3.
- PMID 10831607.
- ISBN 9780393680393.)
{{cite book}}: CS1 maint: location missing publisher (link - ^ ISBN 978-0-7167-2672-2.
- ^ S2CID 172279.
- ^ ISBN 978-0-8153-4072-0.
- PMID 9724605.
- PMID 1429833.
- ^ PMID 11877373.
- PMID 15565881.
- PMID 11121432.
- PMID 11854306.
- PMID 15145358.
- PMID 15182349.
- S2CID 6040822.
- S2CID 9261461.
- PMID 9554838.
- PMID 8947544. Archived from the originalon 29 September 2007.
- PMID 4168731.
- S2CID 30482028.
- S2CID 20769260.
- ^ S2CID 16865665.
- ^ S2CID 8807316.
- ^ PMID 12181345.
- ^ ISBN 978-0-7216-3360-2.
- ^ PMID 11368755.
- PMID 19720872.
- S2CID 29584217.
- ^ PMID 9683623.
- S2CID 44941483.
- PMID 1955462.
- PMID 9501098.
- PMID 15240004.
- PMID 20452955.
- ^
Spector DL, Lamond AI (February 2011). "Nuclear speckles". Review. Cold Spring Harbor Perspectives in Biology. 3 (2): a000646. PMID 20926517.
- ^
Alexander KA, Coté A, Nguyen SC, Zhang L, Berger SL (March 2021). "p53 mediates target gene association with nuclear speckles for amplified RNA expression". Primary. Molecular Cell. 81 (8): S1097-2765(21)00174-X. S2CID 233172170.
- ^ S2CID 6439413.
- (PDF) from the original on 15 November 2011.
- S2CID 6332495.
- PMID 16325406.
- ^ "Cellular component Nucleus speckle". UniProt: UniProtKB. Retrieved 30 August 2013.
- ^
Gall JG, Bellini M, Wu Z, Murphy C (December 1999). "Assembly of the nuclear transcription and processing machinery: Cajal bodies (coiled bodies) and transcriptosomes". Primary. Molecular Biology of the Cell. 10 (12): 4385–402. PMID 10588665.
- ^ S2CID 30268055.
- ^ PMID 20573717.
- ^ a b Fox A, Bickmore W (2004). "Nuclear Compartments: Paraspeckles". Nuclear Protein Database. Archived from the original on 10 September 2008. Retrieved 6 March 2007.
- ^ PMID 16148043.
- PMID 30355755.
- PMID 32072080.
- PMID 17954568.
- ^ PMID 20610547.
- PMID 28424053.
- ISBN 978-1-57259-931-4.
- S2CID 20647022.
- PMID 10611974.
- PMID 14731866.
- ISBN 978-3-527-30638-1.
- ISBN 978-0-7923-4565-7.
- S2CID 23576288.
- ISBN 978-0-8053-9603-4.
- PMID 26793706.
- S2CID 4431000.
- ^ PMID 7549180.
- PMID 23644379.
- PMID 11331311.
- S2CID 44879431.
- ISBN 978-1-119-27891-7.
- PMID 5422968.
- PMID 10708974.
- S2CID 28973947.
- PMID 24368503.
- PMID 9326623.
- PMID 16894968.
- PMID 1779932.
- PMID 23935529.
- PMID 3258008.
- PMID 3027126.
- PMID 22916303.
- S2CID 83769250.
- PMID 24508984.
- PMID 26455774.
- ^ Hogan CM (2010). "Archaea". In Monosson E, Cleveland C (eds.). Encyclopedia of Earth. Washington, DC.: National Council for Science and the Environment. Archived from the original on 11 May 2011.
- ISBN 978-0-7167-1256-5.
- PMID 16242992.
- ^ Bernstein, H., Bernstein, C. (2017). Sexual Communication in Archaea, the Precursor to Eukaryotic Meiosis. In: Witzany, G. (eds) Biocommunication of Archaea. Springer, Cham. https://doi.org/10.1007/978-3-319-65536-9_7
- PMID 16615090.
- PMID 15910279.
- PMID 11805300.
- S2CID 20542871.
- S2CID 21200827.
- PMID 10888648.
- PMID 16846615.
- S2CID 5963228.
- ISBN 978-3-8171-1781-9.
- S2CID 41287948.
- ISBN 978-0-300-07384-3.
- ^ Brown R (1866). "On the Organs and Mode of Fecundation of Orchidex and Asclepiadea". Miscellaneous Botanical Works I: 511–514.
- ^ ISBN 978-3-540-13987-4. Online Version here
Further reading
- Goldman RD, Gruenbaum Y, Moir RD, Shumaker DK, Spann TP (March 2002). "Nuclear lamins: building blocks of nuclear architecture". Genes & Development. 16 (5): 533–47. PMID 11877373.
- A review article about nuclear lamins, explaining their structure and various roles
- Görlich D, Kutay U (1999). "Transport between the cell nucleus and the cytoplasm". Annual Review of Cell and Developmental Biology. 15: 607–60. PMID 10611974.
- A review article about nuclear transport, explains the principles of the mechanism, and the various transport pathways
- Lamond AI, Earnshaw WC (April 1998). "Structure and function in the nucleus" (PDF). Science. 280 (5363): 547–53. PMID 9554838.
- A review article about the nucleus, explaining the structure of chromosomes within the organelle, and describing the nucleolus and other subnuclear bodies
- Pennisi E (August 2004). "Evolutionary biology. The birth of the nucleus". Science. 305 (5685): 766–8. S2CID 83769250.
- A review article about the evolution of the nucleus, explaining a number of different theories
- Pollard TD, Earnshaw WC (2004). Cell Biology. Philadelphia: Saunders. ISBN 978-0-7216-3360-2.
- A university level textbook focusing on cell biology. Contains information on nucleus structure and function, including nuclear transport, and subnuclear domains
External links
- "The Nucleus". MBInfo.
- "Learn about the Cell Nucleus". cellnucleus.com. Website covering structure and function of the nucleus from the Department of Oncology at the University of Alberta.
- Bickmore W. "The Nuclear Protein Database". Medical Research Council Human Genetics Unit. Information on nuclear components.
- "The Nucleus Collection". Image & Video Library. The American Society for Cell Biology. Archived from the original on 12 November 2006. contains peer-reviewed still images and video clips that illustrate the nucleus.
- Gall JG, McIntosh JR (eds.). "Nuclear Envelope and Nuclear Import Section". Landmark Papers in Cell Biology. Archived from the original on 17 November 2006. contains digitized commentaries and links to seminal research papers on the nucleus. Published online in the Image & Video Library Archived 10 June 2011 at the Wayback Machine of The American Society for Cell Biology
- "Cytoplasmic patterns generated by human antibodies". AntibodyPatterns.com. Archived from the original on 2 January 2007.
