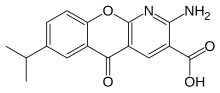
Amlexanox
 | |
| Clinical data | |
|---|---|
| Trade names | Aphthasol |
| AHFS/Drugs.com | Monograph |
| MedlinePlus | a601017 |
| Routes of administration | Topical |
| ATC code | |
| Pharmacokinetic data | |
| Elimination half-life | 3.5 hours |
| Excretion | Renal (17%) |
| Identifiers | |
| |
JSmol) | |
| |
| |
| (verify) | |
Amlexanox (trade name Aphthasol) is an
Medical uses
Amlexanox is the active ingredient in a common topical treatment for recurrent
In Japan, it is used to treat
Contraindications
The drug is contraindicated in those with known allergies to it.[3]
Adverse effects
Amlexanox may cause a slightly painful stinging or burning sensation, nausea or diarrhea.[3]
Mechanism of action
Its mechanism of action is not well-determined, but it might inhibit inflammation by inhibiting the release of
Chemistry
The chemical itself is an odorless, white to yellowish-white powder.[8]
The 5% preparation for patient use is an adherent beige paste,[3][8] and it is also available in some countries as a tablet that adheres to the ulcer in the mouth.[4]
Pharmacokinetics
Amlexanox applied to an aphthous ulcer is largely absorbed through the
History
The patent for its use as a treatment for aphthous ulcers was issued in November 1994 to inventors Kakubhai R. Vora, Atul Khandwala and Charles G. Smith, and assigned to Chemex Pharmaceuticals, Inc.[11]
Society and culture
Economics
A 2011 review found a one-week supply of amlexanox 5% paste to cost $30.[6]
Research
A review found that, as of July 2011[update], robust studies investigating its effectiveness alongside other canker sore treatments were still needed.[12]
Because it is an
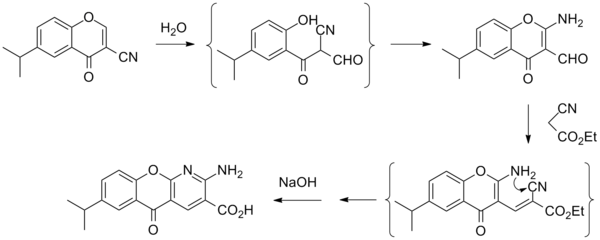
Synthesis
References
- ^ "Amlexanox (Aphthasol®)". Archived from the original on 20 November 2013. Retrieved 20 November 2013.
- PMID 17323710.
- ^ a b c d e "Amlexanox". MedlinePlus. U.S. National Library of Medicine. February 2009. Retrieved 12 February 2013.
- ^ a b Plewa MC (March 2012). "Pediatric Aphthous Ulcers Treatment & Management". Medscape Reference. Medscape. Retrieved 14 February 2013.
- ^ "Amlexanox". PubChem. U.S. National Library of Medicine. Retrieved 12 February 2013.
- ^ PMID 21977491.
- ^ Yousefi M, Ferringer T, Lee S, Bang D (July 2012). "Dermatologic Aspects of Behcet Disease Treatment & Management". Medscape Reference. Medscape. Retrieved 14 February 2013.
- ^ S2CID 24492356.
- ^ PMID 23396211.
- PMID 28683283.
- ^ US patent 5362737, Vora KR, Khandwala A, Smith CG, "Methods of treating aphthous ulcers and other mucocutaneous disorders with amlexanox", published 1994-11-08, assigned to Chemex Pharmaceuticals, Inc.
- PMID 22258979.
- PMID 19737522.
- PMID 3989816.
