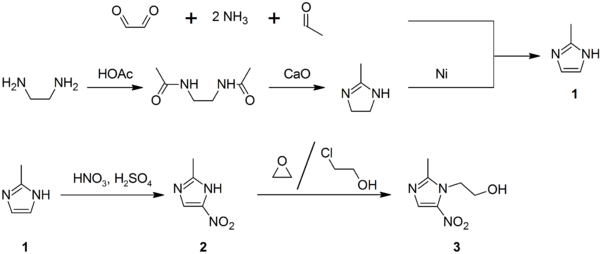Metronidazole
 | |
 | |
| Clinical data | |
|---|---|
| Trade names | Flagyl, Metrogyl |
| AHFS/Drugs.com | Monograph |
| MedlinePlus | a689011 |
| License data | |
| Pregnancy category |
|
QP51CA01 (WHO) | |
| Legal status | |
| Legal status | |
| Pharmacokinetic data | |
| Bioavailability | 80% (by mouth), 60–80% (rectal), 20–25% (vaginal)[8][9][10] |
| Protein binding | 20%[8][9] |
| Metabolism | Liver[8][9] |
| Metabolites | Hydroxymetronidazole |
| Elimination half-life | 8 hours[8][9] |
| Excretion | Urine (77%), faeces (14%)[8][9] |
| Identifiers | |
| |
JSmol) | |
| Melting point | 159 to 163 °C (318 to 325 °F) |
SMILES
| |
| |
| (verify) | |
Metronidazole, sold under the brand name Flagyl among others, is an
Common side effects include
Metronidazole began to be commercially used in 1960 in France.[14] It is on the World Health Organization's List of Essential Medicines.[15] It is available in most areas of the world.[16] In 2022, it was the 133rd most commonly prescribed medication in the United States, with more than 4 million prescriptions.[17][18]
Medical uses

Metronidazole has activity against some
Metronidazole is primarily used to treat:
Metronidazole is bitter and so the liquid suspension contains metronidazole benzoate. This may require hydrolysis in the gastrointestinal tract and some sources speculate that it may be unsuitable in people with diarrhea or feeding-tubes in the duodenum or jejunum.[22][23]
Bacterial vaginosis
Drugs of choice for the treatment of bacterial vaginosis include metronidazole and clindamycin.[24]
An effective treatment option for mixed infectious vaginitis is a combination of clotrimazole and metronidazole.[25]
Trichomoniasis
The
Giardiasis
Oral metronidazole is a treatment option for giardiasis, however, the increasing incidence of nitroimidazole resistance is leading to the increased use of other compound classes.[27]
Dracunculus
In the case of Dracunculus medinensis (Guinea worm), metronidazole merely facilitates worm extraction rather than killing the worm.[11]
C. difficile colitis
Initial antibiotic therapy for less-severe
E. histolytica
Entamoeba histolytica invasive amebiasis is treated with metronidazole for eradication, in combination with diloxanide to prevent recurrence.[29] Although it is generally a standard treatment it is associated with some side effects.[30]
Preterm births
Metronidazole has also been used in women to prevent preterm birth associated with bacterial vaginosis, amongst other risk factors including the presence of cervicovaginal fetal fibronectin (fFN). Metronidazole was ineffective in preventing preterm delivery in high-risk pregnant women (selected by history and a positive fFN test) and, conversely, the incidence of preterm delivery was found to be higher in women treated with metronidazole.[31]
Hypoxic radiosensitizer
In addition to its anti-biotic properties, attempts were also made to use a possible radiation-sensitizing effect of metronidazole in the context of radiation therapy against hypoxic tumors.[32] However, the neurotoxic side effects occurring at the required dosages have prevented the widespread use of metronidazole as an adjuvant agent in radiation therapy.[33] However, other nitroimidazoles derived from metronidazole such as nimorazole with reduced electron affinity showed less serious neuronal side effects and have found their way into radio-onological practice for head and neck tumors in some countries.[34]
Perioral dermatitis
Canadian Family Physician has recommended topical metronidazole as a third-line treatment for the perioral dermatitis either along with or without oral tetracycline or oral erythromycin as first and second line treatment respectively.[35]
Adverse effects
Common
Some evidence from studies in rats indicates the possibility it may contribute to serotonin syndrome, although no case reports documenting this have been published to date.[40][41]
Mutagenesis and carcinogenesis
In 2016 metronidazole was listed by the U.S. National Toxicology Program (NTP) as reasonably anticipated to be a human carcinogen.[42] Although some of the testing methods have been questioned, oral exposure has been shown to cause cancer in experimental animals and has also demonstrated some mutagenic effects in bacterial cultures.[42][43] The relationship between exposure to metronidazole and human cancer is unclear.[42][44] One study [45] found an excess in lung cancer among women (even after adjusting for smoking), while other studies [46][47][48] found either no increased risk, or a statistically insignificant risk.[42][49] Metronidazole is listed as a possible carcinogen according to the World Health Organization (WHO) International Agency for Research on Cancer (IARC).[50] A study in those with Crohn's disease also found chromosomal abnormalities in circulating lymphocytes in people treated with metronidazole.[43]
Stevens–Johnson syndrome
Metronidazole alone rarely causes Stevens–Johnson syndrome, but is reported to occur at high rates when combined with mebendazole.[51]
Neurotoxicity
Several studies in the human[52] and animal models have recorded the neurotoxicity of metronidazole. One possible mechanism underlying this toxicity is that metronidazole may interference with postsynaptic central monoaminergic neurotransmission and immunomodulation.[53] Additionally other research suggests that the role of nitric oxide isoforms and inflammatory cytokines may also play a role.[54]
Drug interactions
Alcohol
Consuming
Other drug interactions
Metronidazole is a moderate inhibitor of the enzyme CYP2C9 belonging to the cytochrome P450 family. As a result, metronidazole may interact with medications metabolized by this enzyme.[59][60][61] Examples of such medications are lomitapide and warfarin, to name a few.[8]
Pharmacology

Mechanism of action
Metronidazole is of the nitroimidazole class. It is a prodrug that inhibits nucleic acid synthesis by forming nitroso radicals, which disrupt the DNA of microbial cells.[8][62] Metronidazole activates by receiving an electron from the reduced ferredoxin produced by pyruvate synthase (PFOR) in anaerobic organisms, equivalent to pyruvate dehydrogenase in aerobic organisms, thus turning into a highly reactive radical anion. After the radical loses the electron to its target, it recycles back to the unactivated form of metronidazole, ready to be activated again.[63]
This function only occurs when metronidazole is partially reduced, and because oxygen competes with metronidazole for the electron, this reduction requires a local environment with low oxygen concentration that usually happens only in anaerobic bacteria and protozoans. Therefore, it has relatively little effect upon human cells or
Pharmacokinetics

Oral metronidazole is approximately 80%
About 60% of the metronidazole is
The biological activity of hydroxymetronidazole is 30% to 65%, and the elimination half-life is longer than that of the parent compound.[65] The serum half-life of hydroxymetronidazole after suppository was 10 hours, 19 hours after intravenous infusion, and 11 hours after a tablet.[66]
Resistance
Resistance in parasites is found in T. vaginilis, and G. lamblia, but not E. histolytica, and two major methods are observed. The first method involves an impaired oxygen scavenging capability that increase the local concentration of oxygen, leading to the decreased activation and increased recycling of metronidazole. The second method is associated with lowered levels of pyruvate synthase and ferredoxin, the latter due to the lowered transcription of the ferredoxin gene. Strains employing the second method will still respond to a higher dosage of metronidazole.[63]
Resistance in bacteria is documented in Bacteriodes spp. that resistant to nitroimidazoles including metronidazole. In the resistant strains, 5-nitroimidazole reductase is identified as the culprit that actively reduces metronidazole to inactive forms. Currently eleven types are identified which are encoded by nimA through nimK respectively. The gene is encoded either in the chromosome or the episome.[63][67][68]
Other mechanisms may include reduced drug activation, efflux pumps, altered redox potential and biofilm formation. In the recent years it is observed that the resistance to metronidazole is increasingly common, complicating its clinical effectiveness.[69][70][71][clarification needed]
History
The drug was initially developed by Rhône-Poulenc in the 1950s[72] and licensed to G.D. Searle.[73] Searle was acquired by Pfizer in 2003.[74] The original patent expired in 1982, but evergreening reformulation occurred thereafter.[75]
Brand name
In India, it is sold under the brand name Metrogyl and Flagyl.[76] In Bangladesh, it is available as Amodis, Amotrex, Dirozyl, Filmet, Flagyl, Flamyd, Metra, Metrodol, Metryl, etc.[77] In Pakistan, it is sold under the brand name of Flagyl and Metrozine.[citation needed] In the United States it is sold under the brand name Noritate.[78]
Synthesis
Research
Metronidazole is researched for its anti-inflammatory and immunomodulatory properties. Studies have shown that metronidazole can decrease the production of
Metronidazole has been studied in various immunological disorders, including inflammatory bowel disease, periodontitis, and rosacea. In these conditions, metronidazole has been suspected to have anti-inflammatory and immunomodulatory effects that could be beneficial in the treatment of these conditions.[86] Despite the success in treating rosacea with metronidazole,[87][88][89][90][91] the exact mechanism of why metronidazole in rosacea is efficient is not precisely known, i.e., which properties of metronidazole help treat rosacea: antibacterial or immunomodulatory or both, or other mechanism is involved.[92][93] Increased ROS production in rosacea is thought to contribute to the inflammatory process and skin damage, so metronidazole's ability to decrease ROS may explain the mechanism of action in this disease, but this remains speculation.[94][95]
Metronidazole is also researched as a potential anti-inflammatory agent in
Veterinary use
Metronidazole is used to treat infections of Giardia in dogs, cats, and other companion animals, but it does not reliably clear infection with this organism and is being supplanted by fenbendazole for this purpose in dogs and cats.[97] It is also used for the management of chronic inflammatory bowel disease, gastrointestinal infections, periodontal disease, and systemic infections in cats and dogs.[98][99] Another common usage is the treatment of systemic and/or gastrointestinal clostridial infections in horses. Metronidazole is used in the aquarium hobby to treat ornamental fish and as a broad-spectrum treatment for bacterial and protozoan infections in reptiles and amphibians. In general, the veterinary community may use metronidazole for any potentially susceptible anaerobic infection. The U.S. Food and Drug Administration (FDA) suggests it only be used when necessary because it has been shown to be carcinogenic in mice and rats, as well as to prevent antimicrobial resistance.[100][101]
The appropriate dosage of metronidazole varies based on the animal species, the condition being treated and the specific formulation of the product.[102]
References
- ^ a b c "Metronidazole Use During Pregnancy". www.drugs.com. Archived from the original on 1 January 2017. Retrieved 1 January 2017.
- FDA. Retrieved 22 October 2023.
- ^ "METRONIDAMED (Medsurge Pharma Pty LTD) | Therapeutic Goods Administration (TGA)". Archived from the original on 15 September 2024. Retrieved 15 September 2024.
- ^ "Flagyl Product information". health-products.canada.ca. 15 May 2024. Retrieved 17 February 2025.
- ^ a b "Metronidazole injection, solution". DailyMed. 16 January 2023. Archived from the original on 6 September 2023. Retrieved 5 July 2023.
- ^ "Metronidazole tablet". DailyMed. 30 January 2023. Archived from the original on 6 September 2023. Retrieved 5 July 2023.
- ^ "Metronidazole Vaginal Gel, 0.75%- metronidazole gel". DailyMed. 17 June 2023. Archived from the original on 6 September 2023. Retrieved 5 July 2023.
- ^ a b c d e f g h "Flagyl, Flagyl ER (metronidazole) dosing, indications, interactions, adverse effects, and more". Medscape Reference. WebMD. Archived from the original on 7 April 2014. Retrieved 3 April 2014.
- ^ a b c d e f Brayfield A, ed. (14 January 2014). "Metronidazole". Martindale: The Complete Drug Reference. Pharmaceutical Press. Retrieved 3 April 2014.[dead link]
- ISBN 978-0-85711-309-2.
- ^ a b c d e f g h "Metronidazole". The American Society of Health-System Pharmacists. Archived from the original on 6 September 2015. Retrieved 31 July 2015.
- ^ PMID 29462280.
- ^ "Safety in Lactation: Metronidazole and tinidazole". SPS - Specialist Pharmacy Service. Archived from the original on 21 February 2020. Retrieved 22 February 2020.
- ISBN 978-1-118-35446-9. Archivedfrom the original on 8 September 2017.
- hdl:10665/371090. WHO/MHP/HPS/EML/2023.02.
- ^ Schmid G (28 July 2003). "Trichomoniasis treatment in women". Archived from the original on 1 August 2015. Retrieved 1 August 2015.
- ^ "The Top 300 of 2022". ClinCalc. Archived from the original on 30 August 2024. Retrieved 30 August 2024.
- ^ "Metronidazole Drug Usage Statistics, United States, 2013 - 2022". ClinCalc. Archived from the original on 6 December 2024. Retrieved 30 August 2024.
- PMID 9360057.
- PMID 20067388.
- ^ ISBN 978-0-9805790-9-3.
- ^ Geoghegan O, Eades C, Moore LS, Gilchrist M (9 February 2017). "Clostridium difficile: diagnosis and treatment update". The Pharmaceutical Journal. Royal Pharmaceutical Society. Archived from the original on 7 March 2019. Retrieved 22 January 2018.
- ISBN 978-0-19-163610-3. Archivedfrom the original on 25 June 2022. Retrieved 29 August 2020.
- PMID 10028110.
- PMID 37773671.
- S2CID 6617019.
- PMID 26258002.
- PMID 17599306.
- OCLC 1004770160.
- S2CID 218475533.
- S2CID 11366650.
- PMID 6884824.
- PMID 24257215.
- PMID 9510041.
- PMID 15856972.
- ^ Side Effects
- ^ "Flagyl metronidazole tablets label" (PDF). Archived (PDF) from the original on 22 January 2016. Retrieved 5 August 2015.
- S2CID 45989669.
- PMID 7986915.
- ^ S2CID 41230661.
- PMID 18971895.
- ^ a b c d National Toxicology Program (2016). "Metronidazole" (PDF). Report on Carcinogens (Fourteenth ed.). National Toxicology Program (NTP). Archived (PDF) from the original on 9 February 2020. Retrieved 9 February 2020.
- ^ a b "Metrogyl Metronidazole Product Information" (PDF). TGA eBusiness Services. Alphapharm Pty Limited. 8 May 2013. Archived from the original on 9 September 2016. Retrieved 3 April 2014.
- PMID 12052431.
- PMID 3339906.
- ^ "Metronidazole (IARC Summary & Evaluation, Supplement7, 1987)". INCHEM2. 3 March 1998. Archived from the original on 4 August 2020. Retrieved 12 September 2019.
- PMID 9762949.
- PMID 19585498.
- ^ "Flagyl 375 U.S. Prescribing Information" (PDF). Pfizer. Archived from the original (PDF) on 7 August 2008.
- ^ "Agents Classified by the IARC Monographs, Volumes 1–124". International Agency for Research on Cancer (IARC). 8 July 2019. Archived from the original on 6 September 2019. Retrieved 12 September 2019.
- PMID 12604501.
- PMID 22870180.
- from the original on 26 July 2024. Retrieved 26 July 2024.
- PMID 32151614.
- PMID 8947362.
- PMID 4320226.
- ^
Williams CS, Woodcock KR (February 2000). "Do ethanol and metronidazole interact to produce a disulfiram-like reaction?". The Annals of Pharmacotherapy. 34 (2): 255–257. S2CID 21151432.
the authors of all the reports presumed the metronidazole-ethanol reaction to be an established pharmacologic fact. None provided evidence that could justify their conclusions
- PMID 12022894.
- ^ Flockhart DA (2007). "Drug Interactions: Cytochrome P450 Drug Interaction Table". Indiana University School of Medicine. Archived from the original on 10 October 2007. Retrieved 4 January 2022.
- S2CID 26910995.
- S2CID 45449460.
- ^ a b c d Haberfeld H, ed. (2020). Austria-Codex (in German). Vienna: Österreichischer Apothekerverlag. Anaerobex-Filmtabletten.
- ^ ISBN 978-0-07-162442-8.
- ^
Eisenstein BI, Schaechter M (2007). "DNA and Chromosome Mechanics". In Schaechter M, Engleberg NC, DiRita VJ, Dermody T (eds.). Schaechter's Mechanisms of Microbial Disease. Hagerstown, MD: Lippincott Williams & Wilkins. p. 28. ISBN 978-0-7817-5342-5.
- S2CID 37891515.
- PMID 6588489.
- PMID 30315962.
- PMID 15492014.
- PMID 35222342.
- S2CID 52983319.
- (PDF) from the original on 18 November 2023. Retrieved 2 February 2024.
- PMID 25557515.
- ^ "G.D. SEARLE & CO. v. COMM | 88 T.C. 252 (1987) | 8otc2521326 | Leagle.com". Leagle. Retrieved 18 June 2019.
- ^ "2003:Pfizer and Pharmacia Merger". Pfizer. Archived from the original on 26 June 2019. Retrieved 18 June 2019.
- PMID 30799664.
- ^ "Metrogyl ER". Medical Dialogues. Archived from the original on 17 April 2021. Retrieved 1 March 2021.
- ^ "Metronidazole Brands". Medex. Archived from the original on 24 November 2021. Retrieved 24 November 2021.
- ^ "Noritate (Metronidazole): Uses, Dosage, Side Effects, Interactions, Warning". RxList. Archived from the original on 8 December 2023. Retrieved 8 December 2023.
- ISBN 978-3-527-30673-2.
- ISBN 978-3-527-30673-2.
- S2CID 38187002.
- S2CID 2267250.
- PMID 26490276.
- S2CID 46474241.
- PMID 20399284.
- S2CID 259126738.
- S2CID 260701677.
- (PDF) from the original on 2 February 2024. Retrieved 2 February 2024.
- PMID 36478427.
- PMID 36177741.
- S2CID 253301872.
- S2CID 218491876.
- PMID 18182250.
- PMID 15499754.
- S2CID 19772010.
- PMID 38613247.
- PMID 7978640.
- ^ Hoskins JD (1 October 2001). "Advances in managing inflammatory bowel disease". DVM Newsmagazine. Archived from the original on 31 December 2013. Retrieved 28 December 2013.
- ^ "METRONIDAZOLE (Veterinary—Systemic)" (PDF). Retrieved 4 July 2024.
- ISBN 978-0-8138-2056-9.
- ^ "Metronidazole". Drugs.com. Archived from the original on 24 June 2013.
- ^ "Metronidazole For Dogs: Safe Dosages And Uses". Forbes. 11 July 2023. Retrieved 4 July 2024.
External links
- "Metronidazole and Tinidazole". Merck manuals.