Nitrosylsulfuric acid

| |

| |
| Names | |
|---|---|
| IUPAC name
Nitrosylsulfuric acid
| |
| Other names
nitrosonium bisulfate, chamber crystals
| |
| Identifiers | |
3D model (
JSmol ) |
|
| ChemSpider | |
ECHA InfoCard
|
100.029.058 |
PubChem CID
|
|
| UNII | |
CompTox Dashboard (EPA)
|
|
| |
| |
| Properties | |
| HSO4NO | |
| Molar mass | 127.08 g/mol |
| Appearance | Pale yellow crystals[1] |
| Density | 1.865 g/mL in 40% sulfuric acid soln [2] |
| Melting point | 70 °C (158 °F; 343 K)[1] |
| Boiling point | Decomposes |
| Decomposes | |
| Solubility | Soluble in H2SO4[1] |
| Hazards | |
| Occupational safety and health (OHS/OSH): | |
Main hazards
|
Oxidizer |
| Related compounds | |
Other anions
|
NOCl |
Other cations
|
NaHSO4 |
Related compounds
|
NOBF4
|
Except where otherwise noted, data are given for materials in their standard state (at 25 °C [77 °F], 100 kPa).
| |
Nitrosylsulfuric acid is the
anhydride of sulfuric acid and nitrous acid
.
In organic chemistry, it is used as a reagent for
diazotizing agent, and as an oxidizing agent.[1]
Synthesis and reactions
A typical procedure entails dissolving sodium nitrite in cold sulfuric acid:[4][5]
- HNO2 + H2SO4 → HSO4NO + H2O
It can also be prepared by the reaction of nitric acid and sulfur dioxide.[6]
HSO4NO is used in
diazonium salts from amines, for example in the Sandmeyer reaction. Related NO-delivery reagents include nitrosonium tetrafluoroborate [NO]+[BF4]− and nitrosyl chloride
.
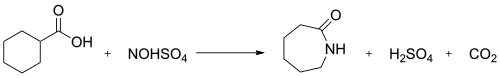
In industry, the nitrosodecarboxylation reaction between nitrosylsulfuric acid and cyclohexanecarboxylic acid is used to generate caprolactam:[3]
Safety
Nitrosylsulfuric acid is a hazardous material and precautions are indicated.[1]
References
- ^ ISBN 978-0471936237.)
{{cite book}}:|journal=ignored (help)CS1 maint: multiple names: authors list (link - ^ "Nitrosylsulfuric acid solution". Merck.
- ^ ISBN 978-3527306732.
- ^ Hodgson, H. H.; Mahadevan, A. P.; Ward, E. R. (1955). "1,4-Dinitronaphthalene". Organic Syntheses; Collected Volumes, vol. 3, p. 341. (diazodization followed by treatment with nitrite)
- ^ Sandin, R. B.; Cairns, T. L. (1943). "1,2,3-Triiodo-5-nitrobenzene". Organic Syntheses; Collected Volumes, vol. 2, p. 604. (diazodization followed by treatment with iodide)
- ISBN 9780470132326. This procedure generates the nitrosylsulfuric acid as an intermediate en route to NOCl.