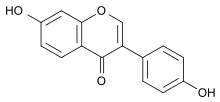
Daidzein
| |

| |
| Names | |
|---|---|
| IUPAC name
4′,7-Dihydroxyisoflavone
| |
| Systematic IUPAC name
7-Hydroxy-3-(4-hydroxyphenyl)-4H-1-benzopyran-4-one | |
| Other names
7-Hydroxy-3-(4-hydroxyphenyl)chromen-4-one
Daidzeol Isoaurostatin | |
| Identifiers | |
3D model (
JSmol ) |
|
| ChEBI | |
| ChEMBL | |
| ChemSpider | |
ECHA InfoCard
|
100.006.942 |
IUPHAR/BPS |
|
| KEGG | |
PubChem CID
|
|
| UNII | |
CompTox Dashboard (EPA)
|
|
| |
| |
| Properties | |
| C15H10O4 | |
| Molar mass | 254.23 g/mol |
| Appearance | Pale yellow prisms |
| Melting point | 315 to 323 °C (599 to 613 °F; 588 to 596 K) (decomposes) |
Except where otherwise noted, data are given for materials in their standard state (at 25 °C [77 °F], 100 kPa).
| |
Daidzein (7-hydroxy-3-(4-hydroxyphenyl)-4H-chromen-4-one) is a naturally occurring compound found exclusively in soybeans and other
Natural occurrence
Daidzein and other isoflavone compounds, such as genistein, are present in a number of plants and herbs like kwao krua (Pueraria mirifica) and kudzu. It can also be found in Maackia amurensis cell cultures.[4] Daidzein can be found in food such as soybeans and soy products like tofu and textured vegetable protein. Soy isoflavones are a group of compounds found in and isolated from the soybean. Of note, total isoflavones in soybeans are—in general—37 percent daidzein, 57 percent genistein and 6 percent glycitein, according to USDA data.[5] Soy germ contains 41.7 percent daidzein.[6]
Biosynthesis
History
The isoflavonoid pathway has long been studied because of its prevalence in a wide variety of plant species, including as pigmentation in many flowers, as well as serving as signals in plants and microbes. The isoflavone synthase (IFS) enzyme was suggested to be a P-450 oxygenase family, and this was confirmed by Shinichi Ayabe's laboratory in 1999. IFS exists in two isoforms that can use both liquiritigenin and naringenin to give daidzein and genistein respectively.[7]
Pathway
Daidzein is an isoflavonoid derived from the shikimate pathway that forms an oxygen containing heterocycle through a cytochrome P-450-dependent enzyme that is NADPH dependent.
The biosynthesis of daidzein begins with L-phenylalanine and undergoes a general phenylpropanoid pathway where the shikimate derived aromatic ring is shifted to the adjacent carbon of the heterocycle.
A radical mechanism has been proposed in order to obtain daidzein from liquiritigenin, where an iron-containing enzyme, as well as NADPH and oxygen cofactors are used by a 2-hydroxyisoflavone synthase to oxidize liquiritigenin to a radical intermediate (C). A 1,2 aryl migration follows to form (D), which is subsequently oxidized to (E). Lastly, dehydration of the hydroxy group on C2 occurs through a

Research
Daidzein has been found to act as an agonist of the GPER (GPR30).[9]
Pathogen interactions
Because daidzein is a defensive factor,
Derivatives
- Glyceollin, a type of phytoalexin[10]
Glycosides
Plants containing daidzein
- Maackia amurensis
- Pueraria montana var. lobata [11][12]
- Pueraria thomsonii [13]
References
- ^ Merck Index, 11th Edition, 2805.
- ^ S2CID 1717934.)
{{cite journal}}: CS1 maint: multiple names: authors list (link - S2CID 245670986.
- PMID 10925005.
- ^ "Isoflavones contents of food". Top Cultures. Retrieved 15 May 2012.
- PMID 10222386.
- ^ PMID 11402179.
- ^ OCLC 259265604.
- PMID 24530924.
- ^ PMID 34414650.
- PMID 11510548.
- .
- PMID 17655152.
