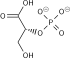
Dihydrolipoyl transacetylase
| DLAT | |||
|---|---|---|---|
Gene ontology | |||
| Molecular function | |||
| Cellular component | |||
| Biological process | |||
| Sources:Amigo / QuickGO | |||
Ensembl | |||||||||
|---|---|---|---|---|---|---|---|---|---|
| UniProt | |||||||||
| RefSeq (mRNA) | |||||||||
| RefSeq (protein) |
| ||||||||
| Location (UCSC) | Chr 11: 112.02 – 112.06 Mb | Chr 9: 50.55 – 50.57 Mb | |||||||
| PubMed search | [3] | [4] | |||||||
| View/Edit Human | View/Edit Mouse |
Dihydrolipoyl transacetylase (or dihydrolipoamide acetyltransferase) is an
There are three different enzyme components in the pyruvate dehydrogenase complex.
In humans, dihydrolipoyl transacetylase enzymatic activity resides in the pyruvate dehydrogenase complex component E2 (PDCE2) that is encoded by the DLAT (dihydrolipoamide S-acetyltransferase) gene.[5]
Nomenclature
The systematic name of this enzyme class is acetyl-CoA:enzyme N6-(dihydrolipoyl)lysine S-acetyltransferase.
Other names in common use include:
- acetyl-CoA:dihydrolipoamide S-acetyltransferase,
- acetyl-CoA:enzyme 6-N-(dihydrolipoyl)lysine S-acetyltransferase.
- dihydrolipoamide S-acetyltransferase,
- dihydrolipoate acetyltransferase,
- dihydrolipoic transacetylase,
- dihydrolipoyl acetyltransferase,
- enzyme-dihydrolipoyllysine:acetyl-CoA S-acetyltransferase,
- lipoate acetyltransferase,
- lipoate transacetylase,
- lipoic acetyltransferase,
- lipoic acid acetyltransferase,
- lipoic transacetylase,
- lipoylacetyltransferase,
- thioltransacetylase A, and
- transacetylase X.
Structure

All dihydrolipoyl transacetylases have a unique multidomain structure consisting of (from N to C): 3 lipoyl domains, an interaction domain, and the catalytic domain (see the domain architecture at Pfam). All the domains are connected by disordered, low complexity linker regions.
Depending on the species, multiple subunits of dihydrolipoyl transacetylase enzymes can arrange together into either a cubic or dodecahedral shape. These structure then form the catalytic core of the pyruvate dehydrogenase complex which not only catalyzes the reaction that transfers an acetyl group to CoA, but also performs a crucial structural role in creating the architecture of the overall complex.[7]
Cube
The cubic core structure, found in species such as Azotobacter vinelandii, is made up of 24 subunits total.[8][9] The catalytic domains are assembled into trimers with the active site located at the subunit interface. The topology of this trimer active site is identical to that of chloramphenicol acetyltransferase. Eight of these trimers are then arranged into a hollow truncated cube. The two main substrates, CoA and the lipoamide (Lip(SH)2), are found at two opposite entrances of a 30 Å long channel which runs between the subunits and forms the catalytic center. CoA enters from the inside of the cube, and the lipoamide enters from the outside.[10]
Dodecahedron
In many species, including bacteria such as
Function
| dihydrolipoyllysine-residue acetyltransferase | |||||||||
|---|---|---|---|---|---|---|---|---|---|
| Identifiers | |||||||||
ExPASy | NiceZyme view | ||||||||
| KEGG | KEGG entry | ||||||||
| MetaCyc | metabolic pathway | ||||||||
| PRIAM | profile | ||||||||
| PDB structures | RCSB PDB PDBe PDBsum | ||||||||
| Gene Ontology | AmiGO / QuickGO | ||||||||
| |||||||||
Dihydrolipoyl transacetylase participates in the pyruvate decarboxylation reaction that links glycolysis to the citric acid cycle. These metabolic processes are important for cellular respiration—the conversion of biochemical energy from nutrients into
Mechanism

Pyruvate decarboxylation requires a few cofactors in addition to the enzymes that make up the complex. The first is thiamine pyrophosphate (TPP), which is used by pyruvate dehydrogenase to oxidize pyruvate and to form a hydroxyethyl-TPP intermediate. This intermediate is taken up by dihydrolipoyl transacetylase and reacted with a second lipoamide cofactor to generate an acetyl-dihydrolipoyl intermediate, releasing TPP in the process. This second intermediate can then be attacked by the nucleophilic sulfur attached to Coenzyme A, and the dihydrolipoamide is released. This results in the production of acetyl CoA, which is the end goal of pyruvate decarboxylation. The dihydrolipoamide is taken up by dihydrolipoyl dehydrogenase, and with the additional cofactors FAD and NAD+, regenerates the original lipoamide (with NADH as a useful side product).
Interactive pathway map
Click on genes, proteins and metabolites below to link to respective articles.[§ 1]
- ^ The interactive pathway map can be edited at WikiPathways: "GlycolysisGluconeogenesis_WP534".
Clinical significance
Primary biliary cirrhosis
Pyruvate dehydrogenase deficiency
Pyruvate dehydrogenase deficiency (PDH) is a genetic disease resulting in lactic acidosis as well as neurological dysfunction in infancy and early childhood. Typically PDH is the result of a mutation in the X-linked gene for the E1 subunit of the pyruvate dehydrogenase complex. However, there have been a few rare cases in which a patient with PDH actually has a mutation in the autosomal gene for the E2 subunit instead. These patients have been reported to have much less severe symptoms, with the most prominent disease manifestation being episodic dystonia, though both hypotonia and ataxia were also present.[16]
References
- ^ a b c GRCh38: Ensembl release 89: ENSG00000150768 – Ensembl, May 2017
- ^ a b c GRCm38: Ensembl release 89: ENSMUSG00000000168 – Ensembl, May 2017
- ^ "Human PubMed Reference:". National Center for Biotechnology Information, U.S. National Library of Medicine.
- ^ "Mouse PubMed Reference:". National Center for Biotechnology Information, U.S. National Library of Medicine.
- PMID 8102256.
- PMID 8471601.
- ^ PMID 9990008.
- PMID 9655933.
- PMID 2917567.
- PMID 1549782.
- PMID 19240034.
- PMID 11752427.
- S2CID 596338. Archived from the originalon 2013-01-05.
- PMID 20180236.
- S2CID 5400712.
- S2CID 38264402.
Further reading
- Mattevi A, Obmolova G, Kalk KH, Teplyakov A, Hol WG (Apr 1993). "Crystallographic analysis of substrate binding and catalysis in dihydrolipoyl transacetylase (E2p)". Biochemistry. 32 (15): 3887–901. PMID 8471601.
- Brady RO, Stadtman ER (Dec 1954). "Enzymatic thioltransacetylation". The Journal of Biological Chemistry. 211 (2): 621–9. PMID 13221570.
- .
- Perham RN (2000). "Swinging arms and swinging domains in multifunctional enzymes: catalytic machines for multistep reactions". Annual Review of Biochemistry. 69: 961–1004. PMID 10966480.
- Howard MJ, Fuller C, Broadhurst RW, Perham RN, Tang JG, Quinn J, Diamond AG, Yeaman SJ (Jul 1998). "Three-dimensional structure of the major autoantigen in primary biliary cirrhosis". Gastroenterology. 115 (1): 139–46. PMID 9649469.
- Matsumura S, Kita H, He XS, Ansari AA, Lian ZX, Van De Water J, Yamamoto K, Tsuji T, Coppel RL, Kaplan M, Gershwin ME (Nov 2002). "Comprehensive mapping of HLA-A0201-restricted CD8 T-cell epitopes on PDC-E2 in primary biliary cirrhosis". Hepatology. 36 (5): 1125–34. S2CID 20687454.
- Suzuki Y, Yoshitomo-Nakagawa K, Maruyama K, Suyama A, Sugano S (Oct 1997). "Construction and characterization of a full length-enriched and a 5'-end-enriched cDNA library". Gene. 200 (1–2): 149–56. PMID 9373149.
- Korotchkina LG, Patel MS (Feb 2008). "Binding of pyruvate dehydrogenase to the core of the human pyruvate dehydrogenase complex". FEBS Letters. 582 (3): 468–72. PMID 18206651.
- Head RA, Brown RM, Zolkipli Z, Shahdadpuri R, King MD, Clayton PT, Brown GK (Aug 2005). "Clinical and genetic spectrum of pyruvate dehydrogenase deficiency: dihydrolipoamide acetyltransferase (E2) deficiency". Annals of Neurology. 58 (2): 234–41. S2CID 38264402.
- Bogdanos DP, Pares A, Baum H, Caballeria L, Rigopoulou EI, Ma Y, Burroughs AK, Rodes J, Vergani D (Jun 2004). "Disease-specific cross-reactivity between mimicking peptides of heat shock protein of Mycobacterium gordonae and dominant epitope of E2 subunit of pyruvate dehydrogenase is common in Spanish but not British patients with primary biliary cirrhosis". Journal of Autoimmunity. 22 (4): 353–62. S2CID 6619201.
- Lleo A, Selmi C, Invernizzi P, Podda M, Coppel RL, Mackay IR, Gores GJ, Ansari AA, Van de Water J, Gershwin ME (Mar 2009). "Apotopes and the biliary specificity of primary biliary cirrhosis" (PDF). Hepatology. 49 (3): 871–9. PMID 19185000.
- Bellucci R, Oertelt S, Gallagher M, Li S, Zorn E, Weller E, Porcheray F, Alyea EP, Soiffer RJ, Munshi NC, Gershwin ME, Ritz J (Mar 2007). "Differential epitope mapping of antibodies to PDC-E2 in patients with hematologic malignancies after allogeneic hematopoietic stem cell transplantation and primary biliary cirrhosis". Blood. 109 (5): 2001–7. PMID 17068145.
- Hiromasa Y, Roche TE (Sep 2003). "Facilitated interaction between the pyruvate dehydrogenase kinase isoform 2 and the dihydrolipoyl acetyltransferase". The Journal of Biological Chemistry. 278 (36): 33681–93. PMID 12816949.
- Trynka G, Zhernakova A, Romanos J, Franke L, Hunt KA, Turner G, Bruinenberg M, Heap GA, Platteel M, Ryan AW, de Kovel C, Holmes GK, Howdle PD, Walters JR, Sanders DS, Mulder CJ, Mearin ML, Verbeek WH, Trimble V, Stevens FM, Kelleher D, Barisani D, Bardella MT, McManus R, van Heel DA, Wijmenga C (Aug 2009). "Coeliac disease-associated risk variants in TNFAIP3 and REL implicate altered NF-kappaB signalling". Gut. 58 (8): 1078–83. S2CID 17111427.
- Hiromasa Y, Fujisawa T, Aso Y, Roche TE (Feb 2004). "Organization of the cores of the mammalian pyruvate dehydrogenase complex formed by E2 and E2 plus the E3-binding protein and their capacities to bind the E1 and E3 components". The Journal of Biological Chemistry. 279 (8): 6921–33. PMID 14638692.
- Cori CF (1981). "The glucose-lactic acid cycle and gluconeogenesis". Current Topics in Cellular Regulation. 18: 377–87. PMID 7273846.
- Maruyama K, Sugano S (Jan 1994). "Oligo-capping: a simple method to replace the cap structure of eukaryotic mRNAs with oligoribonucleotides". Gene. 138 (1–2): 171–4. PMID 8125298.
- Tuganova A, Boulatnikov I, Popov KM (Aug 2002). "Interaction between the individual isoenzymes of pyruvate dehydrogenase kinase and the inner lipoyl-bearing domain of transacetylase component of pyruvate dehydrogenase complex". The Biochemical Journal. 366 (Pt 1): 129–36. PMID 11978179.
External links
- PDB: 1EAA, PDB: 1dpb
- Dihydrolipoyl+transacetylase at the U.S. National Library of Medicine Medical Subject Headings (MeSH)
- Overview of all the structural information available in the PDB for UniProt: P10515 (Dihydrolipoyl transacetylase) at the PDBe-KB.