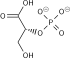
Fructose-bisphosphate aldolase
| Fructose-bisphosphate aldolase | |||||||||
|---|---|---|---|---|---|---|---|---|---|
ExPASy NiceZyme view | | ||||||||
| KEGG | KEGG entry | ||||||||
| MetaCyc | metabolic pathway | ||||||||
| PRIAM | profile | ||||||||
| PDB structures | RCSB PDB PDBe PDBsum | ||||||||
| Gene Ontology | AmiGO / QuickGO | ||||||||
| |||||||||
| Fructose-bisphosphate aldolase class-I | |||||||||
|---|---|---|---|---|---|---|---|---|---|
| CDD | cd00344 | ||||||||
| |||||||||
| Fructose-bisphosphate aldolase class-II | |||||||||
|---|---|---|---|---|---|---|---|---|---|
| CDD | cd00453 | ||||||||
| |||||||||
Fructose-bisphosphate aldolase (
The word aldolase also refers, more generally, to an enzyme that performs an aldol reaction (creating an aldol) or its reverse (cleaving an aldol), such as Sialic acid aldolase, which forms sialic acid. See the list of aldolases.
Mechanism and structure
Class I proteins form a
The
With few exceptions only class I proteins have been found in
In gluconeogenesis and glycolysis
Gluconeogenesis and glycolysis share a series of six reversible reactions. In gluconeogenesis glyceraldehyde-3-phosphate is reduced to fructose 1,6-bisphosphate with aldolase. In glycolysis fructose 1,6-bisphosphate is made into glyceraldehyde-3-phosphate and dihydroxyacetone phosphate through the use of aldolase. The aldolase used in gluconeogenesis and glycolysis is a cytoplasmic protein.
Three forms of class I protein are found in vertebrates.
In the Calvin cycle
The
Reactions
Aldolase catalyzes
- fructose 1,6-bisphosphate ⇌ DHAP + G3P
and also
- sedoheptulose 1,7-bisphosphate ⇌ DHAP + erythrose 4-phosphate
- fructose 1-phosphate ⇌ DHAP + glyceraldehyde
Aldolase is used in the reversible trunk of gluconeogenesis/glycolysis
- 2(PEP+ NADH + H+ + ATP + H2O) ⇌ fructose 1,6-bisphosphate + 2(NAD+ + ADP + Pi)
Aldolase is also used in the part of the Calvin cycle shared with gluconeogenesis, with the irreversible phosphate hydrolysis at the end catalyzed by fructose 1,6-bisphosphatase
- 2(3-PG+ NADPH + H+ + ATP + H2O) ⇌ fructose 1,6-bisphosphate + 2(NADP+ + ADP + Pi)
- fructose 1,6-bisphosphate + H2O → fructose 6-phosphate + Pi
In gluconeogenesis 3-PG is produced by enolase and phosphoglycerate mutase acting in series
- PEP + H2O ⇌ 2-PG ⇌ 3-PG
In the Calvin cycle 3-PG is produced by RuBisCO
- RuBP + CO2 + H2O → 2(3-PG)
G3P is produced by
- 3-PG + ATP ⇌ 1,3-bisphosphoglycerate + ADP
- 1,3-bisphosphoglycerate + NAD(P)H + H+ ⇌ G3P + Pi + NAD(P)+
- G3P ⇌ DHAP
Thus both DHAP and G3P are available to aldolase.
Moonlighting properties
Aldolase has also been implicated in many "moonlighting" or non-catalytic functions, based upon its binding affinity for many other proteins including
References
- PMID 10712619.
- PMID 15470245.
- ^ Trung Hieu Pham, Shreesha Rao, Ta-Chih Cheng, Pei-Chi Wang, Shih-Chu Chen, The moonlighting protein fructose 1,6-bisphosphate aldolase as a potential vaccine candidate against Photobacterium damselae subsp. piscicida in Asian sea bass (Lates calcarifer), Developmental & Comparative Immunology,Volume 124,2021,104187,ISSN 0145-305X,https://doi.org/10.1016/j.dci.2021.104187.
- PMID 11387336.
- PMID 9482935.
- PMID 2371280.
- PMID 21169486.
- PMID 20129922.
- PMID 7925012.
- PMID 21307348.
Further reading
- Berry A, Marshall KE (February 1993). "Identification of zinc-binding ligands in the class II fructose-1,6-bisphosphate aldolase of Escherichia coli". FEBS Lett. 318 (1): 11–6. S2CID 7682431.
- Freemont PS, Dunbar B, Fothergill-Gilmore LA (February 1988). "The complete amino acid sequence of human skeletal-muscle fructose-bisphosphate aldolase". Biochem. J. 249 (3): 779–88. PMID 3355497.
- Galkin A, Li Z, Li L, Kulakova L, Pal LR, Dunaway-Mariano D, Herzberg O (2009). "Structural insights into the substrate binding and stereoselectivity of giardia fructose-1,6-bisphosphate aldolase". Biochemistry. 48 (14): 3186–96. PMID 19236002.
- Marsh JJ, Lebherz HG (March 1992). "Fructose-bisphosphate aldolases: an evolutionary history". Trends Biochem. Sci. 17 (3): 110–3. PMID 1412694.
- Perham RN (April 1990). "The fructose-1,6-bisphosphate aldolases: same reaction, different enzymes". Biochem. Soc. Trans. 18 (2): 185–7. PMID 2199259.
External links
 Media related to Fructose-bisphosphate aldolase at Wikimedia Commons
Media related to Fructose-bisphosphate aldolase at Wikimedia Commons- Tolan Laboratory at Boston University