Schwann cell
| Schwann cell | |
|---|---|
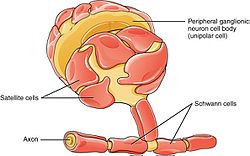 The PNS has satellite cells and Schwann cells. | |
| Identifiers | |
| MeSH | D012583 |
| FMA | 62121 |
| Anatomical terms of neuroanatomy | |
Schwann cells or neurolemmocytes (named after German physiologist
During the development of the PNS, the regulatory mechanisms of myelination are controlled by feedforward interaction of specific genes, influencing transcriptional cascades and shaping the morphology of the myelinated nerve fibers.[2]
Schwann cells are involved in many important aspects of peripheral
Charcot–Marie–Tooth disease, Guillain–Barré syndrome (acute inflammatory demyelinating polyradiculopathy type), schwannomatosis, chronic inflammatory demyelinating polyneuropathy, and leprosy are all neuropathies involving Schwann cells.
Structure
Schwann cells are a variety of
Function
The
Nonmyelinating Schwann cells are involved in maintenance of axons and are crucial for neuronal survival. Some group around smaller axons (External image here) and form
Myelinating Schwann cells begin to form the myelin sheath in mammals during fetal development and work by spiraling around the axon, sometimes with as many as 100 revolutions. A well-developed Schwann cell is shaped like a rolled-up sheet of paper, with layers of myelin between each coil. The inner layers of the wrapping, which are predominantly
Regeneration
Schwann cells are known for their roles in supporting
Schwann cells are essential for the maintenance of healthy axons. They produce a variety of factors, including neurotrophins, and also transfer essential molecules across to axons.

Genetics
Schwann cell formation
Sox10
SOX10 is a transcription factor active during embryonic development and abundant evidence indicates that it is essential for the generation of glial lineages from trunk crest cells.[8][9] When SOX10 is inactivated in mice, satellite glia and Schwann cell precursors fail to develop, though neurons are generated normally without issue.[8] In the absence of SOX10, neural crest cells survive and are free to generate neurons, but glial specification is blocked.[9] SOX10 might influence early glial precursors to respond to neuregulin 1[8] (see below).
Neuregulin 1
Neuregulin 1 (NRG1) acts in a number of ways to both promote the formation and ensure the survival of immature Schwann cells.[10] During embryonic development, NRG1 inhibits the formation of neurons from neural crest cells, instead contributing to neural crest cells being led down a path to gliogenesis. NRG1 signaling is not, however, required for glial differentiation from the neural crest.[11]
NRG1 plays important roles in the development of neural crest derivatives. It is required for neural crest cells to migrate past the site of dorsal root ganglia to find the ventral regions of sympathetic gangliogenesis.[12] It is also an essential axon-derived survival factor and a mitogen for Schwann cell precursors.[13] It is found in the dorsal root ganglion and motor neurons at the point in time that Schwann cell precursors begin to populate spinal nerves and therefore influences Schwann cell survival.[11] In embryonic nerves, the transmembrane III isoform likely is the primary variant of NRG1 responsible for survival signals. In mice that lack the transmembrane III isoform, Schwann cell precursors are eventually eliminated from spinal nerves.[14]
Formation of myelin sheath
P0
Myelin protein zero (P0) is a cell-adhesion molecule belonging to the immunoglobulin superfamily and is the major component of peripheral myelin, constituting over 50% of the total protein in the sheath.[15][16] P0 has been shown to be essential for the formation of compact myelin, as P0 null mutant (P0-) mice showed severely aberrant peripheral myelination.[17] Although myelination of large caliber axons was initiated in P0- mice, the resulting myelin layers were very thin and poorly compacted. Unexpectedly, P0- mice also showed degeneration of both axons and their surround myelin sheaths, suggesting that P0 plays a role in maintaining the structural integrity of both myelin formation and the axon with which it is associated. P0- mice developed behavioral deficits around 2 weeks of age when mice began to show signs of slight trembling. Gross incoordination also arose as the animals developed, while trembling became more severe and some older mice developed convulsing behaviors. Despite the array of impaired motor behavior, no paralysis was observed in these animals. P0 is also an important gene expressed early within the Schwann cell lineage, expressed in Schwann cell precursors after differentiating from migrating neural crest cells within the developing embryo.[18]
Krox-20
Several important transcription factors are also expressed and involved at various stages in development changing the features on the Schwann cells from an immature to mature state. One indispensable transcription factor expressed during the myelination process is Krox-20. It is a general zinc-finger transcription factor and is expressed in the rhombomeres 3 and 5.
Krox-20 is considered one of the master regulators of PNS myelination and is important in driving transcription of specific structural proteins in the myelin. It has been shown to control a set of genes responsible for interfering with this feature in the axon changing it from a pro-myelinating to myelinating state.[19] In this way, in Krox-20 double knock out mice, it has been recorded that hindbrain segmentation is affected as well as myelination of Schwann cell associated axons. Indeed, in these mice, the Schwann cells are not able to perform their myelination properly as they only wrap their cytoplasmic processes one and half turn around the axon and despite the fact that they still express the early myelin marker, late myelin gene products are absent. In addition, recent studies have also proven the importance of this transcription factor in maintaining the myelination phenotype (and requires the co-expression of Sox 10) as its inactivation leads to dedifferentiation of the Schwann cells.[2]
Clinical significance
Transplantation
A number of experimental studies since 2001 have implanted Schwann cells in an attempt to induce remyelination in multiple sclerosis-afflicted patients.[21] In the past two decades, many studies have demonstrated positive results and potential for Schwann cell transplantation as a therapy for spinal cord injury, both in aiding regrowth and myelination of damaged CNS axons.[22] Schwann cell transplants in combination with other therapies such as Chondroitinase ABC have also been shown to be effective in functional recovery from spinal cord injury.[23]
See also
- Electrophysiology
- Hodgkin–Huxley model
- Mesaxon
- Neurotransmission
- Olfactory ensheathing cell
- Perisynaptic schwann cells
- Schwannoma
- List of human cell types derived from the germ layers
- List of distinct cell types in the adult human body
References
- PMID 16807057.
- ^ S2CID 4333028.
- ISBN 978-1-119-32064-7.
- PMID 20739294.
- ^ Kalat, James W. Biological Psychology, 9th ed. USA: Thompson Learning, 2007.[page needed]
- PMID 16807057.
- ^ Carlson, Neil R. Physiology of Behavior, 9th ed. USA: Pearson Education, Inc., 2007.[page needed]
- ^ PMID 11156606.
- ^ PMID 11641219.)
{{cite journal}}: CS1 maint: multiple names: authors list (link - S2CID 20297598.
- ^ S2CID 7540462.
- PMID 9637684.
- S2CID 15332720.
- S2CID 16187922.
- S2CID 30385476.
- S2CID 27086229.
- S2CID 41878912.
- S2CID 7540462.
- PMID 26054742.
- PMID 31289325.
- ^ "First surgical transplant attempted to repair myelin". Inside MS. 2001. Archived from the original on 2007-03-11.
- PMID 16629629.
- PMID 15689553.
External links
- Diagram at clc.uc.edu
- Histology image: 21301loa – Histology Learning System at Boston University—"Ultrastructure of the Cell: myelinated axon and Schwann cell"
- Cell Centered Database—Schwann cell