Birt–Hogg–Dubé syndrome
| Birt–Hogg–Dubé syndrome | |
|---|---|
 | |
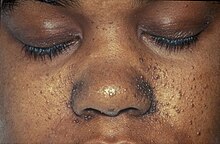
| The characteristic fibrofolliculomas of Birt–Hogg–Dubé syndrome seen on a person's face. | |
| Specialty | Medical genetics |
Birt–Hogg–Dubé syndrome (BHD), also Hornstein–Birt–Hogg–Dubé syndrome, Hornstein–Knickenberg syndrome, and fibrofolliculomas with trichodiscomas and acrochordons
Any of these conditions that occurs in a family can indicate a diagnosis of Birt–Hogg–Dubé syndrome, though it is only confirmed by a
Birt–Hogg–Dubé syndrome can manifest similarly to other diseases, which must be ruled out when making a diagnosis. These include tuberous sclerosis, which causes skin lesions similar to fibrofolliculomas, and Von Hippel–Lindau disease, which causes hereditary kidney cancers. Once diagnosed, people with BHD are treated preventatively, with monitoring of kidneys and lungs using medical imaging. Fibrofolliculomas can be removed surgically and pneumothorax and kidney cancer are treated according to the normal standard of care. Dermatologic examinations, neck ultrasounds and colonoscopies should be considered as well [1].
Signs and symptoms
Skin
Birt–Hogg–Dubé syndrome affects the skin and increases the risk of tumors in the kidneys and lungs. The condition is characterized by multiple noncancerous, dome-shaped tumors of the
Other tumors can include
Kidneys

People over 20 years of age with BHD have an increased risk of developing slow-growing
In general, people with this syndrome are at roughly at seven times the risk of kidney cancer compared to the unaffected population. Estimates of the incidence among people with the disease range from 14 to 34%.
Lungs
Along with fibrofolliculomas and kidney tumors, affected individuals frequently develop
Other organs
Pathophysiology
Genetics

An association with the folliculin (FLCN) gene was first reported in 2002.
Function
FLCN creates a protein, folliculin, that has two
Most of the cancer-causing mutations cause the protein to be truncated at the carboxy terminus.
People with BHD are born with one mutated copy of the FLCN gene in each cell.
Renal cystogenesis and tumorigenesis in BHD have been shown to be driven by the constitutive activation of TFEB.[18]
Diagnosis
BHD can be suggested by clinical findings but is definitively diagnosed by molecular genetic testing to detect mutations in the FLCN gene. The classical clinical triad includes benign growths of the hair follicles, pulmonary cysts and spontaneous pneumothorax, and bilateral, multifocal renal tumors.[5]
Clinical triad
The cutaneous manifestations of BHD were originally described as fibrofolliculomas (abnormal growths of a hair follicle), trichodiscomas (hamartomatous lesions with a hair follicle at the periphery, often found on the face), and acrochordons (skin tags). Cutaneous manifestations are confirmed by
Genetic testing
FLCN mutations are detected by sequencing in 88% of probands with this syndrome. This means that some people with the clinical diagnosis have mutations that are not detectable by current technology, or that mutations in another currently unknown gene could be responsible for a minority of cases. In addition, amplifications and deletions in exonic regions are also tested. Genetic testing can be useful to confirm the clinical diagnosis and to provide a means of determining other at-risk individuals in a family even if they have not yet developed BHD symptoms.[5][6]
Differential diagnosis
BHD can be difficult to diagnose from symptoms alone, because hereditary renal cancers, pneumothorax, and cutaneous tumors occur with other syndromes. Hereditary bilateral, multifocal kidney tumors similar to those seen in BHD can occur with
Hereditary recurrent pneumothorax or pulmonary cysts are associated with
Though fibrofolliculomas are unique to BHD, they may present with an ambiguous appearance and must be confirmed histologically. Other diseases can mimic the dermatologic manifestations of BHD, including tuberous sclerosis complex,
Management
The different manifestations of BHD are controlled in different ways. The fibrofolliculomas can be removed surgically, through
Epidemiology
The disorder has been reported in more than 100 families worldwide,
Patient registry
Birt-Hogg-Dubé Syndrome patients, families, and caregivers are encouraged to join the NIH Rare Lung Diseases Consortium Contact Registry. This is a privacy-protected site that provides up-to-date information for individuals interested in the latest scientific news, trials, and treatments related to rare lung diseases.
History
The syndrome was first well described in 1977,[21] by three Canadian physicians, Arthur R. Birt, Georgina R. Hogg, and William J. Dubé. The earliest case of possible BHD in the medical literature was published by Burnier and Rejsek in 1927,[22] who described a case of perifollicular fibromas on a 56-year-old woman's face. Trichodiscomas were first described in 1974 by H. S. Zackheim and H. Pinkus, but were not associated with BHD until Birt, Hogg, and Dubé.[3] The first case of BHD with the systemic symptoms was described by Hornstein and Knickenberg and found in two siblings and their father, all of whom exhibited colon polyps and the characteristic fibrofolliculomas.[23] Though the siblings did not have renal or pulmonary symptoms, their father had cysts in his lungs and kidneys.[3] Hornstein-Knickenberg syndrome is a now-deprecated name for the inherited fibrofolliculomas inherent to BHD.[5]
Birt, Hogg, and Dubé examined a family with a hereditary thyroid cancer, and discovered that many of the members had fibrofolliculomas, trichodiscomas, and acrochordons, which became defined as the classical symptoms of the eponymous disease. The first case of spontaneous pneumothorax associated with BHD was discovered in 1986;
Other animals
Genes related to FLCN and diseases similar to BHD have been found in dogs, fruit flies, rats, and mice. In German Shepherd dogs, missense mutations in the canine
A homolog of FLCN called DBHD has been discovered in the common fruit fly, Drosophila melanogaster.[27][3] Decrease expression of the DBHD results in loss of male germline stem cells (GSC), which suggest that DBHD is required for male GSC maintenance in the fly testis.[28] Further, DBHD regulates GSC maintenance downstream or in parallel of the JAK/STAT and Dpp signal-transduction pathways, which suggest that BHD regulates tumorigenesis by controlling stem cells in human {[29] Singh et al. 2006}
A line of rats with hereditary kidney cancer were developed by Japanese researchers. They have a mutation in the FLCN homolog that produces a truncated protein, though they do not develop the cutaneous or pulmonary symptoms seen in humans. Heterozygotes have renal abnormalities seen very early in life that develop into clear-cell and hybrid tumors, significantly shortening the animals' lifespans; they also are prone to endometrial and salivary gland clear-cell hyperplasia as well as rhabdomyolysis. Homozygotes do not survive to birth.[3] When a wild-type FLCN gene was added, the phenotype was rescued.[6]
References
Citations
- ^ a b c Genetics Home Reference.
- ^ a b c d e f g h i j k l m n o Andrews 2011.
- ^ a b c d e f g h i j k l m n o p Reese et al. 2009.
- ^ a b c d e f Palmirotta et al. 2010.
- ^ a b c d e f g h i j k l m n o p q r s t u v w x y Toro 2008.
- ^ a b c d e f g h i j k l m n o p q r Menko et al. 2009.
- ^ a b Chan-Smutko 2012, p. 345.
- ^ a b c d e f g Coleman & Russo 2009, p. 482.
- ^ a b Furuya & Nakatani 2012.
- ^ Grant, Babar & Griffin 2009, p. 442.
- ^ Devine & Garcia 2012, p. 4.
- ^ Coleman & Russo 2009, p. 481.
- ^ Nickerson et al. 2002.
- ^ a b c d e Toro et al. 2008.
- ^ "Rare Lung Diseases Disorder Definitions". www1.rarediseasesnetwork.org. Retrieved 13 November 2021.
- ^ a b Maher 2011.
- ^ Sudarshan et al. 2013.
- S2CID 220289684.
- ^ Ayo et al. 2007.
- ^ Verine et al. 2010.
- ^ Birt, Hogg & Dubé 1977.
- ^ Riegert-Johnson.
- ^ Kniffin 2012.
- ^ BHD Foundation.
- ^ National Organization for Rare Disorders.
- ^ Genetics Home Reference: Educational Resources.
- ^ Liu et al. 2013.
- ^ Singh SR, Zhen W, Zheng Z, Wang H, Oh SW, Liu W, Zbar B, Schmidt LS, Hou SX. The Drosophila homolog of the human tumor suppressor gene BHD interacts with the JAK-STAT and Dpp signaling pathways in regulating male germline stem cell maintenance. Oncogene. 2006 Sep 28;25(44):5933-41.
- ^ Singh SR, Zhen W, Zheng Z, Wang H, Oh SW, Liu W, Zbar B, Schmidt LS, Hou SX. The Drosophila homolog of the human tumor suppressor gene BHD interacts with the JAK-STAT and Dpp signaling pathways in regulating male germline stem cell maintenance. Oncogene. 2006 Sep 28;25(44):5933-41
Bibliography
- Ayo, Dereje S.; Aughenbaugh, GL; Yi, ES; Hand, JL; Ryu, JH (2007), "Cystic Lung Disease in Birt-Hogg-Dubé Syndrome", Chest, 132 (2): 679–84, PMID 17505035
- Birt, A. R.; Hogg, GR; Dubé, WJ (1977), "Hereditary multiple fibrofolliculomas with trichodiscomas and acrochordons", Archives of Dermatology, 113 (12): 1674–7, PMID 596896
- BHD Foundation, 2013, archived from the original on 11 June 2021, retrieved 17 July 2013
- Chan-Smutko, Gayun (2012), "Genetic Testing by Cancer Site", The Cancer Journal, 18 (4): 343–9, PMID 22846736
- Coleman, Jonathan A; Russo, Paul (2009), "Hereditary and familial kidney cancer", Current Opinion in Urology, 19 (5): 478–85, PMID 19584731
- Devine, Megan Stuebner; Garcia, Christine Kim (2012), "Genetic Interstitial Lung Disease", Clinics in Chest Medicine, 33 (1): 95–110, PMID 22365249
- Furuya, M.; Nakatani, Y. (2012), "Birt-Hogg-Dube syndrome: Clinicopathological features of the lung", Journal of Clinical Pathology, 66 (3): 178–86, PMID 23223565
- "Birt-Hogg-Dubé syndrome", Genetics Home Reference, US National Library of Medicine, January 2013, retrieved 13 July 2013
- "Birt-Hogg-Dubé syndrome: Educational resources", Genetics Home Reference, NIH, 22 July 2013, archived from the original on 15 March 2016, retrieved 25 July 2013
- Grant, L.A.; Babar, J.; Griffin, N. (2009), "Cysts, cavities, and honeycombing in multisystem disorders: Differential diagnosis and findings on thin-section CT", Clinical Radiology, 64 (4): 439–48, PMID 19264190
- James, William D.; Berger, Timothy; Elston, Dirk (2011), Andrew's Diseases of the Skin: Clinical Dermatology (11th ed.), Elsevier Health Sciences, ISBN 978-1-4377-3619-9
- Kniffin, Cassandra L. (22 August 2012), "Birt-Hogg-Dube syndrome, BHD", Online Mendelian Inheritance in Man, Johns Hopkins University, archived from the original on 10 March 2017, retrieved 13 July 2013
- Liu, Wei; Chen, Zhi; Ma, Yansen; Wu, Xiaochun; Jin, Yaping; Hou, Steven (2013), White-Cooper, Helen (ed.), "Genetic Characterization of the Drosophila Birt-Hogg-Dubé Syndrome Gene", PLOS ONE, 8 (6): e65869, PMID 23799055
- Maher, Eamonn R. (2011), "Genetics of Familial Renal Cancers", Nephron Experimental Nephrology, 118 (1): e21–6, S2CID 23464317
- Menko, Fred H; Van Steensel, Maurice AM; Giraud, Sophie; Friis-Hansen, Lennart; Richard, Stéphane; Ungari, Silvana; Nordenskjöld, Magnus; Hansen, Thomas vO; Solly, John; Maher, Eamonn R; European Bhd, Consortium (2009), "Birt-Hogg-Dubé syndrome: Diagnosis and management", The Lancet Oncology, 10 (12): 1199–206, PMID 19959076
- BHD Foundation, National Organization for Rare Disorders, 2013, archived from the original on 3 November 2013, retrieved 25 July 2013
- Nickerson, Michael L.; Warren, Michelle B.; Toro, Jorge R.; Matrosova, Vera; Glenn, Gladys; Turner, Maria L.; Duray, Paul; Merino, Maria; Choyke, Peter; et al. (2002), "Mutations in a novel gene lead to kidney tumors, lung wall defects, and benign tumors of the hair follicle in patients with the Birt-Hogg-Dubé syndrome", Cancer Cell, 2 (2): 157–64, PMID 12204536
- Palmirotta, Raffaele; Savonarola, Annalisa; Ludovici, Giorgia; Donati, Pietro; Cavaliere, Francesco; De Marchis, Maria Laura; Ferroni, Patrizia; Guadagni, Fiorella (March 2010), "Association between Birt Hogg Dubé syndrome and cancer predisposition", Anticancer Res., 30 (3): 751–7, PMID 20392993
- Reese, Erin; Sluzevich, Jason; Kluijt, Irma; Teertstra, H. Jelle; De Jong, Daphne; Horenblas, Simon; Ryu, Jay (5 October 2009), "Birt-Hogg-Dubé Syndrome", in Riegert-Johnson, Douglas L; Boardman, Lisa A; Hefferon, Timothy; Roberts, Maegan (eds.), Cancer Syndromes, Bethesda, MD: National Center for Biotechnology Information, PMID 21249760
- Riegert-Johnson, DL, "Birt-Hogg-Dube", Familial Cancer Syndromes, NCBI, retrieved 21 July 2009
- Sudarshan, Sunil; Karam, Jose A.; Brugarolas, James; Thompson, R. Houston; Uzzo, Robert; Rini, Brian; Margulis, Vitaly; Patard, Jean-Jacques; Escudier, Bernard; Linehan, W. Marston (2013), "Metabolism of Kidney Cancer: From the Lab to Clinical Practice", European Urology, 63 (2): 244–51, PMID 23063455
- Toro, Jorge R. (9 September 2008), "Birt-Hogg-Dubé Syndrome", in Pagon, Roberta A; Adam, Margaret P; Bird, Thomas D; Dolan, Cynthia R; Fong, Chin-To; Smith, Richard JH; Stephens, Karen (eds.), GeneReviews, University of Washington, PMID 20301695
- Toro, J R; Wei, M-H; Glenn, G M; Weinreich, M; Toure, O; Vocke, C; Turner, M; Choyke, P; Merino, M J; et al. (2008), "BHD mutations, clinical and molecular genetic investigations of Birt-Hogg-Dube syndrome: A new series of 50 families and a review of published reports", Journal of Medical Genetics, 45 (6): 321–31, PMID 18234728
- Verine, Jérôme; Pluvinage, Amélie; Bousquet, Guilhem; Lehmann-Che, Jacqueline; De Bazelaire, Cédric; Soufir, Nadem; Mongiat-Artus, Pierre (2010), "Hereditary Renal Cancer Syndromes: An Update of a Systematic Review", European Urology, 58 (5): 701–10, PMID 20817385
External links


