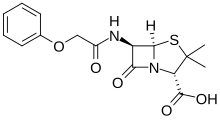
Phenoxymethylpenicillin
 | |
 | |
| Clinical data | |
|---|---|
| Trade names | Veetids, Apocillin,[1] others |
| Other names | penicillin phenoxymethyl, penicillin V, penicillin VK |
| AHFS/Drugs.com | Monograph |
| MedlinePlus | a685015 |
| License data |
|
| Routes of administration | By mouth |
| ATC code | |
| Legal status | |
| Legal status |
|
| Pharmacokinetic data | |
| Bioavailability | 60% |
| Protein binding | 80% |
| Metabolism | Liver |
| Elimination half-life | 30–60 min |
| Excretion | Kidney |
| Identifiers | |
| |
JSmol) | |
| Melting point | 120–128 °C (248–262 °F) |
| |
| |
| (verify) | |
Phenoxymethylpenicillin, also known as penicillin V (PcV) and penicillin VK, is an
Side effects include
Phenoxymethylpenicillin was first made in 1948 by
Medical uses
Specific uses for phenoxymethylpenicillin include:[9][10]
- Infections caused by Streptococcus pyogenes
- Tonsillitis
- Pharyngitis
- Skin infections
- Anthrax (mild uncomplicated infections)
- Lyme disease (early stage in pregnant women or young children)
- Rheumatic fever (primary and secondary prophylaxis)
- Streptococcal skin infections
- Spleen disorders (pneumococcal infection prophylaxis)
- Initial treatment for dental abscesses
- Moderate-to-severe gingivitis (with metronidazole)
- Avulsion injuriesof teeth (as an alternative to tetracycline)
- Blood infection prophylaxis in children with sickle cell disease.
Penicillin V is sometimes used in the treatment of odontogenic infections.[citation needed]
It is less active than benzylpenicillin (penicillin G) against Gram-negative bacteria.[11][12] Phenoxymethylpenicillin has a range of antimicrobial activity against Gram-positive bacteria that is similar to that of benzylpenicillin and a similar mode of action, but it is substantially less active than benzylpenicillin against Gram-negative bacteria.[11][12]
Phenoxymethylpenicillin is more acid-stable than benzylpenicillin, which allows it to be given orally.[citation needed]
Phenoxymethylpenicillin is usually used only for the treatment of mild to moderate infections, and not for severe or deep-seated infections since
It is not active against beta-lactamase-producing bacteria, which include many strains of Staphylococci.[13]
Adverse effects
Phenoxymethylpenicillin is usually well tolerated but may occasionally cause transient nausea, vomiting, epigastric distress, diarrhea, constipation, acidic smell to urine and black hairy tongue. A previous hypersensitivity reaction to any penicillin is a contraindication.[9][13]
Mechanism of action
The mechanism of phenoxymethylpenicillin is identical to that of all other penicillins. It exerts a
Compendial status
History
The Austrian pharmaceutical company, Biochemie, was founded in Kundl in July 1946 at the site of a derelict brewery, at the suggestion of a French officer, Michel Rambaud (a chemist), who was able to obtain a small amount of Penicillium start culture from France. Contamination of the fermentation tanks was a persistent problem and in 1951, the company biologist, Ernst Brandl, attempted to solve this by adding phenoxyethanol to the tanks as an anti-bacterial disinfectant. This resulted unexpectedly in an increase in penicillin production: but, the penicillin produced was not benzylpenicillin, but phenoxymethylpenicillin. Phenoxyethanol was fermented to phenoxyacetic acid[16] in the tanks, which was then incorporated into penicillin via biosynthesis. Importantly, Brandl realised that phenoxymethylpenicillin is not destroyed by stomach acid and can therefore be given by mouth. Phenoxymethyl penicillin was originally discovered by Eli Lilly in 1948 as part of their efforts to study penicillin precursors, but was not further exploited, and there is no evidence that Lilly understood the significance of their discovery at the time.[5]: 119–121 [17]
Biochemie is part of Sandoz.[citation needed]
Society and culture
Names
There were four named penicillins at the time penicillin V was discovered (penicillins I, II, III, IV), however, Penicillin V was named "V" for Vertraulich (German for confidential);[5]: 121 it was not named for the Roman numeral "5". Penicillin VK is the potassium salt of penicillin V (K is the chemical symbol for potassium).[citation needed]
References
- ^ "Apocillin". Felleskatalogen (in Norwegian). LMI (Legemiddelindustrien). Retrieved 23 June 2018.
fenoksymetylpenicillin
- ^ ISBN 9789241547659.
- ISBN 9781284057560.
- ^ a b c "Penicillin V". The American Society of Health-System Pharmacists. Archived from the original on 20 December 2016. Retrieved 8 December 2016.
- ^ ISBN 978-0-19-953484-5. Archivedfrom the original on 20 December 2016.
- hdl:10665/325771. WHO/MVP/EMP/IAU/2019.06. License: CC BY-NC-SA 3.0 IGO.
- ^ "The Top 300 of 2021". ClinCalc. Archived from the original on 15 January 2024. Retrieved 14 January 2024.
- ^ "Penicillin V - Drug Usage Statistics". ClinCalc. Retrieved 14 January 2024.
- ^ a b c Sweetman S., ed. (2002). Martindale: The complete drug reference (Electronic version ed.). London: Royal Pharmaceutical Society of Great Britain and the Pharmaceutical Press.
- ISBN 0-9757919-2-3.
- ^ a b
Garrod LP (February 1960). "Relative antibacterial activity of three penicillins". British Medical Journal. 1 (5172): 527–529. PMID 13826674.
- ^ a b
Garrod LP (December 1960). "The relative antibacterial activity of four penicillins". British Medical Journal. 2 (5214): 1695–1696. PMID 13703756.
- ^ Daily Med: Current Medication Information. December 2006. Archivedfrom the original on 27 July 2009. Retrieved 2 August 2009.
- PMID 7372662.
- ^ "Index (BP 2009)" (PDF). British Pharmacopoeia Commission Secretariat. Archived from the original (PDF) on 11 April 2009. Retrieved 26 March 2010.
- ^ "Phenoxyacetic acid". PubChem. Bethesda, MD, USA: National Center for Biotechnology Information, National Library of Medicine. 26 December 2020. Retrieved 1 January 2021.
- PMID 18880772.
