Connexin
| Connexin | |||||||||
|---|---|---|---|---|---|---|---|---|---|
TCDB 1.A.24 | | ||||||||
| OPM superfamily | 194 | ||||||||
| OPM protein | 2zw3 | ||||||||
| |||||||||
Connexins (Cx) (TC# 1.A.24), or
Nomenclature
Connexins are commonly named according to their molecular weights, e.g. Cx26 is the connexin protein of 26 kDa. A competing nomenclature is the gap junction protein system, where connexins are sorted by their α (GJA) and β (GJB) forms, with additional connexins grouped into the C, D and E groupings, followed by an identifying number, e.g. GJA1 corresponds to Cx43. Following a vote at the Gap Junction Conference (2007) in Elsinore the community agreed to use the GJ nomenclature system for the genes that encode connexins, but wished to retain the connexin nomenclature for the encoded proteins using the weight of the human protein for the numbering of orthologous proteins.
Structure
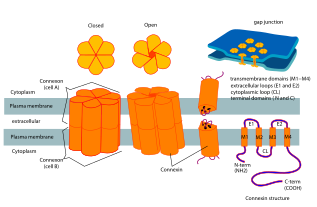
Connexins contain four highly ordered transmembrane segments (TMSs), primarily unstructured C and N cytoplasmic termini, a cytoplasmic loop (CL) and two extra-cellular loops, (EL-1) and (EL-2). Connexins are assembled in groups of six to form hemichannels, or connexons, and two hemichannels then combine to form a gap junction.
The crystal structure of the gap junction channel formed by human Cx26 (also known as GJB2) at 3.5 Å resolution is available.[3] The density map showed the two membrane-spanning hemichannels and the arrangement of the four TMSs of the six protomers forming each hemichannel. The hemichannels feature a positively charged cytoplasmic entrance, a funnel, a negatively charged transmembrane pathway, and an extracellular cavity. The pore is narrowed at the funnel, which is formed by the six amino-terminal helices lining the wall of the channel, which thus determines the molecular size restriction at the channel entrance.
The connexin gene family is diverse, with twenty-one identified members in the sequenced human genome, and twenty in the mouse (nineteen of which are orthologous pairs). They usually weigh between 25 and 60 kDa, and have an average length of 380 amino acids. The various connexins have been observed to combine into both homomeric and heteromeric gap junctions, each of which may exhibit different functional properties including pore conductance, size selectivity, charge selectivity, voltage gating, and chemical gating.[4]
Biosynthesis and internalization
A remarkable aspect of connexins is that they have a relatively short half life of only a few hours.[5] The result is the presence of a dynamic cycle by which connexins are synthesized and replaced. It has been suggested that this short life span allows for more finely regulated physiological processes to take place, such as in the myometrium.
From the nucleus to the membrane
As they are being translated by ribosomes, connexins are inserted into the membrane of the
Gap junction assembly
After being inserted into the plasma membrane of the cell, the hemichannels freely diffuse within the lipid bilayer. suggesting a higher level of coordination than previously thought.
Function
Connexin gap junctions are found only in vertebrates, while a functionally analogous (but genetically unrelated) group of proteins, the innexins, are responsible for gap junctions in invertebrate species. Innexin orthologs have also been identified in Chordates, but they are no longer capable of forming gap junctions. Instead, the channels formed by these proteins (called pannexins) act as very large transmembrane pores that connect the intra- and extracellular compartments.
Within the CNS, gap junctions provide electrical coupling between progenitor cells, neurons, and glial cells. By using specific connexin knockout mice, studies revealed that cell coupling is essential for visual signaling. In the retina, ambient light levels influence cell coupling provided by gap junction channels, adapting the visual function for various lighting conditions. Cell coupling is governed by several mechanisms, including connexin expression.[17]
Decrock et al.. have discussed a multilevel platform via which connexins and pannexins can influence the following cellular functions within a tissue: (1) connexin gap junctional channels (GJCs) enable direct cell-cell communication of small molecules, (2) connexin hemichannels and pannexin channels can contribute to
Substrate specificity
Different connexins may exhibit differing specificities for solutes. For example, adenosine passed about 12-fold better through channels formed by Cx32 while AMP and ADP passed about 8-fold better, and ATP greater than 300-fold better, through channels formed by Cx43. Thus, addition of phosphate to adenosine appears to shift its relative permeability from channels formed by Cx32 to channels formed by Cx43. This may have functional consequence because the energy status of a cell could be controlled via connexin expression and channel formation.[19]
Transport reaction
The transport reaction catalyzed by connexin gap junctions is:
- Small molecules (cell 1 cytoplasm) ⇌ small molecules (cell 2 cytoplasm)
Human connexins and clinical significance
| Connexin | Gene | Location and Function |
|---|---|---|
| Cx43 | GJA1 | Expressed at the surface of vasculature with atherosclerotic plaque, and up-regulated during atherosclerosis in mice. May have pathological effects. Also expressed between granulosa cells, which is required for proliferation. Normally expressed in astrocytes, also detected in most of the human astrocytomas and in the astroglial component of glioneuronal tumors.[20] It is also the main cardiac connexin, found mainly in ventricular myocardium.[21] Associated with oculodentodigital dysplasia .
|
| Cx46 | GJA3 | |
| Cx37 | GJA4 | Induced in vascular smooth muscle during coronary arteriogenesis. Cx37 mutations are not lethal. Forms gap junctions between oocytes and granulosa cells, and are required for oocyte survival. |
| Cx40 | GJA5 | Expressed selectively in atrial myocytes. Responsible for mediating the coordinated electrical activation of atria.[22] |
| Cx33 | GJA6 (GJA6P) |
Pseudogene in humans |
| Cx50 | GJA8 | Gap junctions between A-typ horizontal cells in mouse and rabbit retina[23] |
| Cx59 | GJA10 | |
| Cx62 | GJA10 | Human Cx62 complies Cx57 (mouse). Location in axon-bearing B-typ horizontal cell in rabbit retina[24] |
| Cx32 | GJB1 | Major component of the peripheral myelin. Mutations in the human gene cause X-linked Charcot-Marie-Tooth disease, a hereditary neuropathy. In human normal brain CX32 expressed in neurons and oligodendrocytes.[20]
|
| Cx26 | GJB2 | Mutated in |
| Cx31 | GJB3 | Can be associated with Erythrokeratodermia variabilis. |
| Cx30.3 | GJB4 | Fonseca et al. confirmed Cx30.3 expression in thymocytes.[26] Can be associated with Erythrokeratodermia variabilis. |
| Cx31.1 | GJB5 | |
| Cx30 | GJB6 | Mutated in Clouston syndrome (hidrotic ectodermal dysplasia)[25]
|
| Cx25 | GJB7 | |
| Cx45 | GJC1/GJA7 | Human pancreatic ductal epithelial cells.[27] Atrio-ventricular node. |
| Cx47 | GJC2/GJA12 | Expressed in oligodendrocyte gap junctions[28] |
| Cx31.3 | GJC3 | Human ortholog of murine Cx29. Not known to form gap junctions.[29] |
| Cx36 | GJD2/GJA9 | Pancreatic beta cell function, mediating the release of insulin. central nervous system where they synchronize neural activity.[30]
|
| Cx31.9 | GJD3/GJC1 | |
| Cx39 | GJD4 | |
| Cx40.1 | GJD4 | |
| Cx23 | GJE1
|
Gap junctions are essential for many physiological processes, such as the coordinated depolarization of cardiac muscle, proper embryonic development, and the conducted response in microvasculature. For this reason, deletion or mutation of the various connexin isoforms produces distinctive phenotypes and pathologies.[31] While mutations in Cx43 are mostly linked to oculodentodigital dysplasia, Cx47 mutations are associated with Pelizaeus-Merzbacher-like disease and lymphedema. Cx40 mutations are principally linked to atrial fibrillation. Mutations in Cx37 have not yet been described, but polymorphisms in the Cx37 gene have been implicated in the development of arterial disease.[32][33]
References
- ISBN 0-7167-4366-3.
- ^ PMID 22613178.
- S2CID 4431769.
- PMID 16601118.
- ^ PMID 16492141.
- S2CID 18566176.
- S2CID 12169415.
- PMID 10207897.
- PMID 10085106.
- PMID 9792698.
- PMID 9430718.
- S2CID 3029281.
- S2CID 13486416.
- PMID 1650371.
- S2CID 770387.
- PMID 19284610.
- S2CID 2919282.
- ^ S2CID 17170098.
- PMID 12119284.
- ^ S2CID 6738913.
- PMID 9130448.
- PMID 16790700.
- ISBN 978-1-934115-46-6.
- ISBN 978-1-934115-46-6.
- ^ S2CID 205187359.
- PMID 15234537.
- S2CID 34571252.
- PMID 16203097.
- PMID 18353664.
- PMID 15217338.
- S2CID 24404442.
- PMID 36067749.
- S2CID 10070999.
Sources
- As of this edit, this article uses content from "1.A.24 The Gap Junction-forming Connexin (Connexin) Family", which is licensed in a way that permits reuse under the Creative Commons Attribution-ShareAlike 3.0 Unported License, but not under the GFDL. All relevant terms must be followed.
External links
 Media related to connexins at Wikimedia Commons
Media related to connexins at Wikimedia Commons- Connexins at the U.S. National Library of Medicine Medical Subject Headings (MeSH)
