Diagnosis of multiple sclerosis
This article needs additional citations for verification. (March 2024) |
Current standards for diagnosing multiple sclerosis (MS) are based on the 2018 revision of McDonald criteria. They rely on MRI detection (or clinical demonstration) of demyelinating lesions in the CNS, which are distributed in space (DIS) and in time (DIT). It is also a requirement that any possible known disease that produces demyelinating lesions is ruled out before applying McDonald's criteria.[citation needed]
This last requirement makes MS an ill-defined entity, whose borders change every time that a new disease is set apart. Some cases previously considered MS are now considered distinct conditions, like
Sometimes the diagnosis must be retrospective, relying on gradual worsening of neurological signs/symptoms, due to the lack of understanding of the pathogenicity driving disease progression.[1] However, the only definite diagnosis of MS is post-mortem autopsy, where lesions typical of MS can be detected through histopathological techniques.[2][3]
Overview
Multiple sclerosis is typically diagnosed based on the presenting signs and symptoms, in combination with supporting medical imaging and laboratory testing.[4] It can be difficult to confirm, especially early on, since the signs and symptoms may be similar to those of other medical problems.[5][6]
The McDonald criteria, which focus on clinical, laboratory, and radiologic evidence of lesions at different times and in different areas, is the most commonly used method of diagnosis[7] with the Schumacher and Poser criteria being of mostly historical significance.[8] While the above criteria allow for a non-invasive diagnosis, some state that the only definitive proof is an autopsy or biopsy where lesions typical of MS are detected.[5][2][3]
Clinical data alone may be sufficient for a diagnosis of MS if an individual has had separate episodes of neurologic symptoms characteristic of the disease.
Trends towards earlier diagnosis
Prodomal or early phase
Studies have found increased interactions with health services by people who were diagnosed with MS several years later. The range of symptoms included pain and fatigue. Studies continue on whether MS has a prodromal or early phase, and could possibly be diagnosed and treated much earlier.[12][13][14][15]
DMT effectiveness
A range of disease-modifying treatments (DMT) are now available, to reduce the long-term progression of MS. Studies are increasingly indicating that early and aggressive use of DMTs is beneficial for long-term outcomes.[16][17]
Diagnostic criteria
This section needs additional citations for verification. (March 2024) |
McDonald criteria
As of 2021, the McDonald criteria for MS are the most commonly used.
The 2017 McDonald criteria can be summarize in this table:
| Clinical Presentation | Additional Data Needed |
|---|---|
| * 2 or more attacks (relapses) * 2 or more objective clinical lesions |
None; clinical evidence will suffice (additional evidence desirable but must be consistent with MS) |
| * 2 or more attacks * 1 objective clinical lesion (as well as clear-cut historical evidence of a previous attack involving a lesion in a distinct anatomical location) |
None. |
| * 2 or more attacks * 1 objective clinical lesion |
Dissemination in space, demonstrated by an additional clinical attack implication a different CNS site or by MRI. |
| * 1 attack * 2 or more objective clinical lesions |
Dissemination in time, demonstrated by an additional clinical attack or by MRI
OR Demonstration of CSF-specific oligoclonal bands |
| * 1 attack * 1 objective clinical lesion (monosymptomatic presentation) |
Dissemination in space demonstrated by an additional clinical attack implicating a different CNS site or by MRI. AND Dissemination in time demonstrated by an additional clinical attack or by MRI, OR Demonstration of CSF-specific oligoclonal bands |
| Insidious neurological progression suggestive of MS (primary progressive MS) |
One year of disease progression (retrospectively or prospectively determined) and
Two of the following:
|
Okuda Criteria
These criteria, used mainly for research in MS, define what should be considered a Radiologically Isolated Syndrome (RIS).
Research into diagnostic techniques
Multiple sclerosis diagnosis can only be made when there is proof of lesions disseminated in time and in space. Therefore, when damage in the CNS is big enough to be seen. It would be desirable to make it faster.
The ideal diagnosis schema would be able to determine for any given subject, if he will develop MS, at any point in his life, and when. Nevertheless, not enough is currently known about the MS underlying conditions to achieve that.
In order to get as close as possible to the ideal diagnosis status, a lot of research into
Biomarkers in MS
An active field of research is looking for biomarkers for MS that could speed-up the diagnosis doing it more accurate at the same time. While most of them are still under research, there are some of them already well stablished:
- oligoclonal bands: They present proteins that are in the CNS or in blood. Those that are in CNS but not in blood suggest a diagnosis of MS.
- free light chains (FLC), specially the kappa-FLCs (kFLCs). Several authors have reported that the nephelometric and ELISA FLCs determination is comparable with OCBs as markers of IgG synthesis, and kFLCs behave even better than oligoclonal bands.[21]
Differential diagnosis
Several conditions can mimic MS. Given the unknown pathogenesis of MS, its differential diagnosis is based in exclusion of known conditions.[citation needed]
Very close diseases with similar symptoms are the whole "inflammatory
Outside this spectrum, another important mimic is neuroborreliosis. A Borrelia-specific IgG index exists, and testing for it could make the differential diagnosis.[22]
Clinical courses
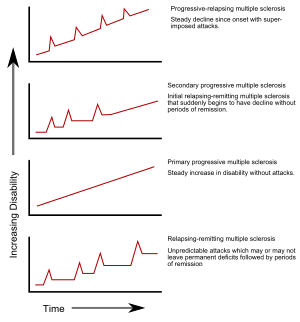
Several phenotypes (commonly named types), or patterns of progression, have been described. Phenotypes use the past course of the disease in an attempt to
The original structure approved in 1996, sometimes still used, was:
- relapsing-remitting
- secondary progressive (SPMS)
- primary progressive (PPMS)
- progressive relapsing.
This set of courses was reviewed by an international panel in 2013,[24][25] adding clinically isolated syndrome (CIS) and radiologically isolated syndrome (RIS) as phenotypes, and finally removing the "progressive relapsing" phenotype. They also added modificators for the clinical courses based on a pair of characteristics: Active/non-active and with/without progression.
The four currently accepted courses or stages are:
- Clinically isolated syndrome (CIS)
- Relapsing-remitting MS (RRMS)
- Primary progressive MS (PPMS)
- Secondary progressive MS (SPMS)
Relapsing-remitting
The relapsing-remitting subtype is characterized by unpredictable relapses followed by periods of months to years of relative quiet (
Secondary progressive

Secondary progressive MS occurs in around 65% of those with initial relapsing-remitting MS, who eventually have progressive neurologic decline between acute attacks without any definite periods of remission.[5][23] Occasional relapses and minor remissions may appear.[23] The most common length of time between disease onset and conversion from relapsing-remitting to secondary progressive MS is 19 years.[29]
Primary progressive
The primary progressive subtype occurs in approximately 10–20% of individuals, with no remission after the initial symptoms.[4][30] It is characterized by progression of disability from onset, with no, or only occasional and minor, remissions and improvements.[23] The usual age of onset for the primary progressive subtype is later than of the relapsing-remitting subtype. It is similar to the age that secondary progressive usually begins in relapsing-remitting MS, around 40 years of age.[5]
Progressive relapsing
Progressive relapsing MS describes those individuals who, from onset, have a steady neurologic decline but also have clear superimposed attacks. This is the least common of all subtypes.[23]
Atypical MS
History
This section needs additional citations for verification. (March 2024) |
Since the first description of multiple sclerosis (MS) by Charcot, the Neurological community has been striving to create reliable and reproducible criteria for diagnosis of MS.[32] The first attempts were made by Charcot himself, followed by Marburg and later Allison. The first criteria however were lacking in sensitivity and specificity for clinical use.[32]
The first landmark event in the history of diagnostic criteria for MS was the development of the Schumacher criteria. These were the first internationally recognized criteria for diagnosis of MS and introduced very important diagnostic concepts that are the cornerstone of MS diagnosis nowadays, such as the clinical definition of MS and the requirement of dissemination in time and space for accurate diagnosis.[citation needed]
Since then, other diagnostic criteria have been proposed. Among them, Poser criteria utilized several laboratory and paraclinical studies to enhance the diagnostic accuracy. McDonald criteria, which are the ones used today, successfully introduced MRI findings as surrogates for the criterion of dissemination in time and space when clinical data are lacking, thus allowing earlier diagnosis of MS.[32]
Historic diagnostic criteria
Schumacher criteria
To get a diagnosis of CDMS a patient must show the following:[33]
- Clinical signs of a problem in the CNS
- Evidence of two or more areas of CNS involvement
- Evidence of white matter involvement
- One of these: Two or more relapses (each lasting ≥ 24 hr and separated by at least 1 month) or progression (slow or stepwise)
- Patient should be between 10 and 50 yr old at time of examination
- No better explanation for patient's symptoms and signs
The last condition, no better explanation for symptoms, has been heavily criticised, but it has been preserved and it is currently included in the new McDonalds criteria in the form that "no better explanation should exist for MRI observations"[This quote needs a citation]
Poser criteria
Poser criteria can be summarized in this table:
Any of the five conclusions have subpossibilities. Here a table is shown with each one of them:
| Clinical Presentation | Additional Data Needed | |
|---|---|---|
| CDMS | * Two or more attacks (relapses) | Two clinical evidence One clinical and one paraclinical evidence |
| LSDMS | * At least one attack and oligoclonal bands | Two attacks and one evidence (clinical or paraclinical) One attack and two clinical evidences One attack, one clinical and one paraclinical evidences |
| CPMS | * At least one attack | Two attacks and one clinical evidence One attack and two clinical evidences One attack, one clinical and one paraclinical evidences |
| LSPMS | * Two attacks | No more evidence is required |
If none of these requirements is fulfilled, the diagnosis is "No MS", meaning that there is not enough clinical evidence to support a clinical diagnosis of MS.
Barkhof-Tintoré criteria
Barkhof criteria,[34] later modified by Tintoré[35] were an early attempt to use MRI to diagnose MS. It was developed by Frederik Barkhof.[34]
Their observations were taken into account when McDonald criteria were published, and therefore they can be considered deprecated by the latter.
References
- .
- ^ S2CID 13870943.
- ^ S2CID 54512368.
- ^ PMID 22146321.
- ^ S2CID 195686659.
- S2CID 3057096.
- ISBN 978-92-4-156375-8.
- S2CID 23452341.
- PMID 18256986.
- S2CID 22724352.
- PMID 10802774.
- PMID 34494923.
- S2CID 214810456.
- S2CID 13691058.
- S2CID 214750591.
- S2CID 237095594.
- S2CID 236997752.
- S2CID 9981947.
- PMID 29452342.
- ^ PMID 26652013.
- ^ Fabio Duranti; Massimo Pieri; Rossella Zenobi; Diego Centonze; Fabio Buttari; Sergio Bernardini; Mariarita Dessi (August 2015). "kFLC Index: a novel approach in early diagnosis of Multiple Sclerosis". International Journal of Scientific Research. 4 (8).
- S2CID 149606491.
- ^ S2CID 40213123.
- PMID 24871874.
- ^ National Multiple Sclerosis Society. "Changes in multiple sclerosis disease-course (or "type") descriptions" (PDF). Archived (PDF) from the original on 3 August 2016. Retrieved 21 August 2017.
NEW COURSE ADDED: Clinically Isolated Syndrome (CIS)...COURSE ELIMINATED: Progressive Relapsing (PRMS).
- PMID 18219812.
- ISBN 978-0-521-85234-0.
- ^ S2CID 36401666.
- S2CID 39503553.
- S2CID 31389841.
- S2CID 21212935.
- ^ S2CID 4498332.
- S2CID 68141248.
- ^ PMID 9397021.
- PMID 10782781.

