Clay

Clay is a type of fine-grained natural soil material containing clay minerals[1] (hydrous aluminium phyllosilicates, e.g. kaolinite, Al2Si2O5(OH)4). Most pure clay minerals are white or light-coloured, but natural clays show a variety of colours from impurities, such as a reddish or brownish colour from small amounts of iron oxide.[2][3]
Clays develop
Clay is a very common substance. Shale, formed largely from clay, is the most common sedimentary rock.[9] Although many naturally occurring deposits include both silts and clay, clays are distinguished from other fine-grained soils by differences in size and mineralogy. Silts, which are fine-grained soils that do not include clay minerals, tend to have larger particle sizes than clays. Mixtures of sand, silt and less than 40% clay are called loam. Soils high in swelling clays (expansive clay), which are clay minerals that readily expand in volume when they absorb water, are a major challenge in civil engineering.[1]
Properties
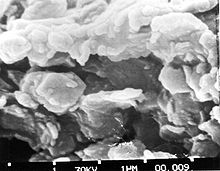
The defining mechanical property of clay is its plasticity when wet and its ability to harden when dried or fired. Clays show a broad range of water content within which they are highly plastic, from a minimum water content (called the plastic limit) where the clay is just moist enough to mould, to a maximum water content (called the liquid limit) where the moulded clay is just dry enough to hold its shape.[10] The plastic limit of kaolinite clay ranges from about 36% to 40% and its liquid limit ranges from about 58% to 72%.[11] High-quality clay is also tough, as measured by the amount of mechanical work required to roll a sample of clay flat. Its toughness reflects a high degree of internal cohesion.[10]
Clay has a high content of clay minerals that give it its plasticity. Clay minerals are
The tiny size and plate form of clay particles gives clay minerals a high surface area. In some clay minerals, the plates carry a negative electrical charge that is balanced by a surrounding layer of positive ions (
Clay is a common component of
Some clay minerals (such as smectite) are described as swelling clay minerals, because they have a great capacity to take up water, and they increase greatly in volume when they do so. When dried, they shrink back to their original volume. This produces distinctive textures, such as mudcracks or "popcorn" texture, in clay deposits. Soils containing swelling clay minerals (such as bentonite) pose a considerable challenge for civil engineering, because swelling clay can break foundations of buildings and ruin road beds.[1]
Formation

Clay minerals most commonly form by prolonged chemical
The clay minerals formed depend on the composition of the source rock and the climate. Acid weathering of feldspar-rich rock, such as granite, in warm climates tends to produce kaolin. Weathering of the same kind of rock under alkaline conditions produces illite. Smectite forms by weathering of igneous rock under alkaline conditions, while gibbsite forms by intense weathering of other clay minerals.[16]
There are two types of clay deposits: primary and secondary. Primary clays form as residual deposits in soil and remain at the site of formation. Secondary clays are clays that have been transported from their original location by water erosion and
Varieties
The main groups of clays include
Varve (or varved clay) is clay with visible annual layers that are formed by seasonal deposition of those layers and are marked by differences in erosion and organic content. This type of deposit is common in former glacial lakes. When fine sediments are delivered into the calm waters of these glacial lake basins away from the shoreline, they settle to the lake bed. The resulting seasonal layering is preserved in an even distribution of clay sediment banding.[14]
Quick clay is a unique type of marine clay indigenous to the glaciated terrains of Norway, North America, Northern Ireland, and Sweden.[22] It is a highly sensitive clay, prone to liquefaction, and has been involved in several deadly landslides.[23]
Uses


Ancient peoples in
| Mass bathing in liquid clay | |
|---|---|
| as a type of relaxation | |
YouTube |

Medicine
Traditional uses of
Construction
Clay as the defining ingredient of
Clay, relatively
See also
- Argillaceous minerals– Fine-grained aluminium phyllosilicates
- Industrial plasticine – Modeling material which is mainly used by automotive design studios
- Clay animation– Stop-motion animation made using malleable clay models
- Clay chemistry – The chemical structures, properties and reactions of clay minerals
- Clay court – Type of tennis court
- Clay panel – Building material made of clay with some additives
- Clay pit – Open-pit mining for the extraction of clay minerals
- Geophagia – Practice of eating earth or soil-like substrates such as clay or chalk
- Graham Cairns-Smith – Scottish chemist known for his controversial book Seven Clues to the Origin of Life
- London Clay – Low-permeable marine geological formation
- Modelling clay – Any of a group of malleable substances used in building and sculpting
- Paper clay – Clay with cellulose fiber
- Particle size – Notion for comparing dimensions of particles in different states of matter
- Plasticine – Brand of modeling clay
- Vertisol – Clay-rich soil, prone to cracking
- Clay–water interaction – Various progressive interactions between clay minerals and water
Notes
- ^ a b c Olive et al. 1989.
- ^ Klein & Hurlbut 1993, pp. 512–514.
- ^ Nesse 2000, pp. 252–257.
- ^ a b Guggenheim & Martin 1995, pp. 255–256.
- ^ Science Learning Hub 2010.
- ^ a b Breuer 2012.
- ^ a b Scarre 2005, p. 238.
- ^ a b Ebert 2011, p. 64.
- ^ a b Boggs 2006, p. 140.
- ^ a b Moreno-Maroto & Alonso-Azcárate 2018.
- ^ White 1949.
- ^ a b Bergaya, Theng & Lagaly 2006, pp. 1–18.
- ^ Hodges 2010.
- ^ a b c Foley 1999.
- ^ Leeder 2011, pp. 5–11.
- ^ Leeder 2011, pp. 10–11.
- ^ Murray 2002.
- ^ Nesse 2000, p. 253.
- ^ Klein & Hurlbut 1993, pp. 514–515.
- ^ Klein & Hurlbut 1993, p. 512.
- ^ Nesse 2000, p. 256.
- ^ Rankka et al. 2004.
- ^ Natural Resources Canada 2005.
- ^ "British Library". www.bl.uk. Archived from the original on 12 September 2022. Retrieved 9 May 2023.
- ^ Forouzan et al. 2012.
- ^ Nesse 2000, p. 257.
- ^ Boylu 2011.
- ^ Eisenhour & Brown 2009.
- ^ Diamond 1999.
- ^ Dadu et al. 2015.
- ^ Grim 2016.
- ^ Koçkar, Akgün & Aktürk 2005.
- ^ García-Sanchez, Alvarez-Ayuso & Rodriguez-Martin 2002.
- ^ Churchman et al. 2006.
References
- Clay mineral nomenclature American Mineralogist.
- Bergaya, Faïza; Theng, B. K. G.; Lagaly, Gerhard (2006). Handbook of Clay Science. Elsevier. ISBN 978-0-08-044183-2.
- Boggs, Sam (2006). Principles of sedimentology and stratigraphy (4th ed.). Upper Saddle River, N.J.: Pearson Prentice Hall. ISBN 0131547283.
- Boylu, Feridun (1 April 2011). "Optimization of foundry sand characteristics of soda-activated calcium bentonite". Applied Clay Science. 52 (1): 104–108. .
- Breuer, Stephen (July 2012). "The chemistry of pottery" (PDF). Education in Chemistry: 17–20. Archived (PDF) from the original on 9 October 2022. Retrieved 8 December 2020.
- Churchman, G. J.; Gates, W. P.; Theng, B. K. G.; Yuan, G. (2006). Faïza Bergaya, Benny K. G. Theng and Gerhard Lagaly (ed.). "Chapter 11.1 Clays and Clay Minerals for Pollution Control". Developments in Clay Science. Handbook of Clay Science. 1. Elsevier: 625–675. ISBN 9780080441832.
- Dadu, Ramona; Hu, Mimi I.; Cleeland, Charles; Busaidy, Naifa L.; Habra, Mouhammed; Waguespack, Steven G.; Sherman, Steven I.; Ying, Anita; Fox, Patricia; Cabanillas, Maria E. (October 2015). "Efficacy of the Natural Clay, Calcium Aluminosilicate Anti-Diarrheal, in Reducing Medullary Thyroid Cancer–Related Diarrhea and Its Effects on Quality of Life: A Pilot Study". Thyroid. 25 (10): 1085–1090. PMID 26200040.
- Diamond, Jared M. (1999). "Diamond on Geophagy". ucla.edu. Archived from the original on 28 May 2015.
- Ebert, John David (31 August 2011). The New Media Invasion: Digital Technologies and the World They Unmake. McFarland. ISBN 9780786488186. Archivedfrom the original on 24 December 2017.
- Ehlers, Ernest G. and Blatt, Harvey (1982). 'Petrology, Igneous, Sedimentary, and Metamorphic' ISBN 0-7167-1279-2.
- Eisenhour, D. D.; Brown, R. K. (1 April 2009). "Bentonite and Its Impact on Modern Life". Elements. 5 (2): 83–88. .
- Foley, Nora K. (September 1999). "Environmental Characteristics of Clays and Clay Mineral Deposits". usgs.gov. Archived from the original on 8 December 2008.
- Forouzan, Firoozeh; Glover, Jeffrey B.; Williams, Frank; Deocampo, Daniel (1 December 2012). "Portable XRF analysis of zoomorphic figurines, "tokens," and sling bullets from Chogha Gavaneh, Iran". Journal of Archaeological Science. 39 (12): 3534–3541. .
- García-Sanchez, A.; Alvarez-Ayuso, E.; Rodriguez-Martin, F. (1 March 2002). "Sorption of As(V) by some oxyhydroxides and clay minerals. Application to its immobilization in two polluted mining soils". Clay Minerals. 37 (1): 187–194. S2CID 101864343.
- Grim, Ralph (2016). "Clay mineral". Encyclopædia Britannica. Archived from the original on 9 December 2015. Retrieved 10 January 2016.
- Guggenheim, Stephen; Martin, R. T. (1995), "Definition of clay and clay mineral: Journal report of the AIPEA nomenclature and CMS nomenclature committees", Clays and Clay Minerals, 43 (2): 255–256, S2CID 129312753
- Hillier S. (2003) "Clay Mineralogy." pp 139–142 In Middleton G.V., Church M.J., Coniglio M., Hardie L.A. and Longstaffe F.J. (Editors) Encyclopedia of Sediments and Sedimentary Rocks. Kluwer Academic Publishers, Dordrecht.
- Hodges, S.C. (2010). "Soil fertility basics" (PDF). Soil Science Extension, North Carolina State University. Archived (PDF) from the original on 9 October 2022. Retrieved 8 December 2020.
- Klein, Cornelis; Hurlbut, Cornelius S. Jr. (1993). Manual of mineralogy : (after James D. Dana) (21st ed.). New York: Wiley. ISBN 047157452X.
- Koçkar, Mustafa K.; Akgün, Haluk; Aktürk, Özgür (November 2005). "Preliminary evaluation of a compacted bentonite / sand mixture as a landfill liner material (Abstract)]". Department of Geological Engineering, Middle East Technical University, Ankara, Turkey. Archived from the original on 4 December 2008.
- Leeder, M. R. (2011). Sedimentology and sedimentary basins : from turbulence to tectonics (2nd ed.). Chichester, West Sussex, UK: Wiley-Blackwell. ISBN 978-1-40517783-2.
- Moreno-Maroto, José Manuel; Alonso-Azcárate, Jacinto (September 2018). "What is clay? A new definition of "clay" based on plasticity and its impact on the most widespread soil classification systems". Applied Clay Science. 161: 57–63. S2CID 102760108.
- Murray, H. (2002). "Industrial clays case study" (PDF). Mining, Minerals and Sustainable Development. 64: 1–9. Archived from the original (PDF) on 20 April 2021. Retrieved 8 December 2020.
- "Landslides". Geoscape Ottawa-Gatineau. Natural Resources Canada. 7 March 2005. Archived from the original on 24 October 2005. Retrieved 21 July 2016.
- Nesse, William D. (2000). Introduction to mineralogy. New York: Oxford University Press. ISBN 9780195106916.
- Olive, W.W.; Chleborad, A.F.; Frahme, C.W.; Shlocker, Julius; Schneider, R.R.; Schuster, R.L. (1989). "Swelling Clays Map of the Conterminous United States". U.S. Geological Survey Miscellaneous Investigations Series Map. I-1940. Retrieved 8 December 2020.
- Rankka, Karin; Andersson-Sköld, Yvonne; Hultén, Carina; Larsson, Rolf; Leroux, Virginie; Dahlin, Torleif (2004). "Quick clay in Sweden" (PDF). Report No. 65. Swedish Geotechnical Institute. Archived from the original (PDF) on 4 April 2005. Retrieved 20 April 2005.
- Scarre, C. (2005). The Human Past. London: Thames and Hudson. ISBN 0500290636.
- "What is clay". Science Learning Hub. University of Waikato. Archived from the original on 3 January 2016. Retrieved 10 January 2016.
- White, W.A. (1949). "Atterberg plastic limits of clay minerals" (PDF). American Mineralogist. 34 (7–8): 508–512. Archived (PDF) from the original on 9 October 2022. Retrieved 7 December 2020.
