PRKACA
Ensembl | |||||||||
|---|---|---|---|---|---|---|---|---|---|
| UniProt | |||||||||
| RefSeq (mRNA) | |||||||||
| RefSeq (protein) | |||||||||
| Location (UCSC) | Chr 19: 14.09 – 14.12 Mb | Chr 8: 84.7 – 84.72 Mb | |||||||
| PubMed search | [3] | [4] | |||||||
| View/Edit Human | View/Edit Mouse |
The catalytic subunit α of protein kinase A is a key regulatory
Discovery
Another key event in the history of PKA occurred when Susan Taylor and Janusz Sowadski at the
Catalytic subunits
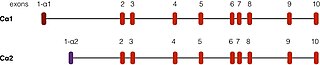
PRKACA is found on
In addition, there are two other isoforms of the catalytic subunit of PKA called Cβ and Cγ arising from different genes but have similar functions as Cα.[13][14] Cβ is found abundantly in the brain and in lower levels in other tissues, while Cγ is most likely expressed in the testis.
Signaling

Inactive PKA holoenzyme exists as a tetramer composed of two regulatory (R) subunits and two catalytic (C) subunits.[15] Biochemical studies demonstrated that there are two types of R subunits. The type I R subunits of which there are two isoforms (RIα, and RIβ) bind the catalytic subunits to create the type I PKA holoenzyme. Likewise type II R subunits, of which there are two isoforms (RIIα, and RIIβ), create the type II PKA holoenzyme. In the presence of cAMP, each R subunit binds 2 cAMP molecules and causes a conformational change in the R subunits that releases the C subunits to phosphorylate downstream substrates.[16] The different R subunits differ in their sensitivity to cAMP, expression levels and subcellular locations. A-kinase-anchoring proteins (AKAPs) bind a surface formed between both R subunits and target the kinase to different locations in the cell. This optimizes where and when cellular communication occurs within the cell.[11]
Clinical significance
Protein kinase A has been implicated in a number of diseases, including cardiovascular disease, tumors of the adrenal cortex, and cancer. It has been speculated that abnormally high levels of PKA phosphorylation contributes to heart disease. This affects excitation-contraction coupling, which is a rhythmic process that controls the contraction of cardiac muscle through the synchronized actions of calcium and cAMP responsive enzymes.[17] There is also evidence to support that the mis-localization of PKA signaling contributes to cardiac arrhythmias, specifically Long QT syndrome. This results in irregular heartbeats that can cause sudden death.
Mutations in the PRKACA gene that promote abnormal enzyme activity have been linked to disease of the adrenal gland. Several mutations in PRKACA have been found in patients with
There is recent and growing interest in
Notes
Wikidata Q34505964 . |
References
- ^ a b c ENSG00000288516 GRCh38: Ensembl release 89: ENSG00000072062, ENSG00000288516 – Ensembl, May 2017
- ^ a b c GRCm38: Ensembl release 89: ENSMUSG00000005469 – Ensembl, May 2017
- ^ "Human PubMed Reference:". National Center for Biotechnology Information, U.S. National Library of Medicine.
- ^ "Mouse PubMed Reference:". National Center for Biotechnology Information, U.S. National Library of Medicine.
- ^ PMID 8884279.
- PMID 190238.
- PMID 11181538.
- PMID 15718245.
- PMID 13252012.
- PMID 1862342.
- ^ PMID 25785716.
- PMID 23593352.
- PMID 3023318.
- PMID 2342480.
- PMID 38740.
- PMID 20368369.
- PMID 16645149.
- S2CID 22892253.
- PMID 24578576.
External links
- PDBe-KB provides an overview of all the structure information available in the PDB for Human cAMP-dependent protein kinase catalytic subunit alpha (PRKACA)
