Canavanine
| |
| Names | |
|---|---|
| Preferred IUPAC name
Canavanine | |
| Systematic IUPAC name
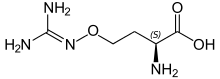
(2S)-2-amino-4-{[(diaminomethylidene)amino]oxy}butanoic acid | |
| Identifiers | |
3D model (
JSmol ) |
|
| ChEBI | |
| ChemSpider | |
| DrugBank | |
ECHA InfoCard
|
100.153.281 |
| EC Number |
|
| KEGG | |
| MeSH | Canavanine |
PubChem CID
|
|
| UNII | |
CompTox Dashboard (EPA)
|
|
| |
| |
| Properties | |
| C5H12N4O3 | |
| Molar mass | 176.176 g·mol−1 |
| Density | 1.61 g·cm−3 (predicted) |
| Melting point | 184 °C (363 °F; 457 K) |
| Boiling point | 366 °C (691 °F; 639 K) |
| soluble | |
| Solubility | insoluble in alcohol, ether, benzene |
| log P | -0.91 (predicted) |
| Vapor pressure | 1.61 μPa (predicted) |
| Acidity (pKa) | 2.35 (carboxylic acid), 7.01 (oxoguanidinium), 9.22 (ammonium) |
| Hazards | |
| GHS labelling: | |

| |
| Warning | |
| H302, H312, H332 | |
| Flash point | 214.6 °C (418.3 °F; 487.8 K) (predicted) |
Except where otherwise noted, data are given for materials in their standard state (at 25 °C [77 °F], 100 kPa).
| |
L-(+)-(S)-Canavanine is a
Toxicity
The mechanism of canavanine's toxicity is that organisms that consume it typically mistakenly incorporate it into their own proteins in place of L-arginine, thereby producing structurally aberrant proteins that may not function properly. Cleavage by arginase also produces canaline, a potent insecticide.
The toxicity of canavanine may be enhanced under conditions of protein starvation,

In mammals
NZB/W F1, NZB, and DBA/2 mice fed L-canavanine develop a syndrome similar to
Tolerance
Some specialized herbivores tolerate L-canavanine either because they metabolize it efficiently (cf. L-canaline) or avoid its incorporation into their own nascent proteins.
By metabolic detoxification
Herbivores may be able to metabolize canavanine efficiently. The beetle Caryedes brasiliensis is able to break canavanine down to canaline, then further detoxifies canaline by reductive deamination to form homoserine and ammonia. As a result, the beetle not only tolerates the chemical, but uses it as a source of nitrogen to synthesize its other amino acids to allow it to develop.[7]
By selectivity
An example of this ability can be found in the larvae of the tobacco budworm
See also
References
- ^ PMID 16890899.
- PMID 15733319.
- PMID 1862241.
- ]
- ^ http://vegpeace.org/rawfoodtoxins.html[full citation needed][permanent dead link][unreliable medical source?]
- .
- .
- PMID 9122181.
- PMID 3455753.
- S2CID 26741233.
This article needs additional citations for verification. (January 2011) |
Bibliography
- Rosenthal, Gerald A. (1986). "Biochemical insight into insecticidal properties ofl-Canavanine, a higher plant protective allelochemical". Journal of Chemical Ecology. 12 (5): 1145–56. S2CID 37297952.
- Rosenthal, G. A.; Berge, M. A.; Bleiler, J. A.; Rudd, T. P. (1987). "Aberrant, canavanyl protein formation and the ability to tolerate or utilize L-canavanine". Experientia. 43 (5): 558–61. S2CID 24239284.
- Boyar, A.; Marsh, R. E. (1982). "L-Canavanine, a paradigm for the structures of substituted guanidines". Journal of the American Chemical Society. 104 (7): 1995–1998. .
- Turner BL, Harborne JB (1967). "Distribution of canavanine in the plant kingdom". Phytochemistry. 6 (6): 863–866. doi:10.1016/S0031-9422(00)86033-1. and in particularly large amounts in Canavalia gladiata(sword bean).
- Ekanayake S, Skog K, Asp NG (May 2007). "Canavanine content in sword beans (Canavalia gladiata): analysis and effect of processing". Food and Chemical Toxicology. 45 (5): 797–803. PMID 17187914.
- Ekanayake S, Skog K, Asp NG (May 2007). "Canavanine content in sword beans (Canavalia gladiata): analysis and effect of processing". Food and Chemical Toxicology. 45 (5): 797–803.
.........
