Hydrogel
| Polymer science |
|---|
 |


A hydrogel is a biphasic material, a mixture of porous, permeable solids and at least 10% by weight or volume of interstitial fluid composed completely or mainly by water.[1][2] In hydrogels the porous permeable solid is a water insoluble three dimensional network of natural or synthetic polymers and a fluid, having absorbed a large amount of water or biological fluids.[2][3][4][5] These properties underpin several applications, especially in the biomedical area. Many hydrogels are synthetic, but some are derived from nature.[6][7] The term 'hydrogel' was coined in 1894.[8]

Chemistry
Classification
The crosslinks which bond the polymers of a hydrogel fall under two general categories: physical hydrogels and chemical hydrogels. Chemical hydrogels have
Hydrogels are prepared using a variety of
Preparation

There are two suggested mechanisms behind physical hydrogel formation, the first one being the gelation of nanofibrous peptide assemblies, usually observed for oligopeptide precursors. The precursors self-assemble into fibers, tapes, tubes, or ribbons that entangle to form non-covalent cross-links. The second mechanism involves non-covalent interactions of cross-linked domains that are separated by water-soluble linkers, and this is usually observed in longer multi-domain structures.[21] Tuning of the supramolecular interactions to produce a self-supporting network that does not precipitate, and is also able to immobilize water which is vital for to gel formation. Most oligopeptide hydrogels have a β-sheet structure, and assemble to form fibers, although α-helical peptides have also been reported.[22][23] The typical mechanism of gelation involves the oligopeptide precursors self-assemble into fibers that become elongated, and entangle to form cross-linked gels.
One notable method of initiating a polymerization fuving involves the use of light as a stimulus. In this method,
Physically crosslinked hydrogels can be prepared by different methods depending on the nature of the crosslink involved. Polyvinyl alcohol hydrogels are usually produced by the freeze-thawed technique. In this, the solution is frozen for a few hours, then thawed at room temperature, and the cycle is repeated until a strong and stable hydrogel is formed.[26] Alginate hydrogels are formed by ionic interactions between alginate and double-charged cations. A salt, usually calcium chloride, is dissolved into an aqueous sodium alginate solution, that causes the calcium ions to create ionic bonds between alginate chains.[27] Gelatin hydrogels are formed by temperature change. A water solution of gelatin forms an hydrogel at temperatures below 37–35 °C, as Van der Waals interactions between collagen fibers become stronger than thermal molecular vibrations.[28]
Peptides based hydrogels
Peptides based hydrogels possess exceptional
Other
Hydrogels also possess a degree of flexibility very similar to natural tissue due to their significant water content. As responsive "
-
-
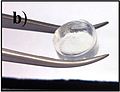
A short-peptide-based hydrogel matrix, capable of holding about one hundred times its own weight in water. Developed as a medical dressing.
-
Photo of the same short-peptide-based hydrogel, held in forceps to demonstrate its stiffness and transparency.
Mechanical properties
Hydrogels have been investigated for diverse applications. By modifying the polymer concentration of a hydrogel (or conversely, the water concentration), the
Hydrogels have two main regimes of mechanical properties: rubber elasticity and viscoelasticity:
Rubber elasticity
In the unswollen state, hydrogels can be modelled as highly crosslinked chemical gels, in which the system can be described as one continuous polymer network. In this case:
where G is the shear modulus, k is the Boltzmann constant, T is temperature, Np is the number of polymer chains per unit volume, ρ is the density, R is the ideal gas constant, and is the (number) average molecular weight between two adjacent cross-linking points. can be calculated from the swell ratio, Q, which is relatively easy to test and measure.[40]
For the swollen state, a perfect gel network can be modeled as:[40]
In a simple uniaxial extension or compression test, the true stress, , and engineering stress, , can be calculated as:
where is the stretch.[40]
Viscoelasticity
For hydrogels, their elasticity comes from the solid polymer matrix while the viscosity originates from the polymer network mobility and the water and other components that make up the aqueous phase.[42] Viscoelastic properties of a hydrogel is highly dependent on the nature of the applied mechanical motion. Thus, the time dependence of these applied forces is extremely important for evaluating the viscoelasticity of the material.[43]
Physical models for viscoelasticity attempt to capture the elastic and viscous material properties of a material. In an elastic material, the stress is proportional to the strain while in a viscous material, the stress is proportional to the strain rate. The Maxwell model is one developed mathematical model for linear viscoelastic response. In this model, viscoelasticity is modeled analogous to an electrical circuit with a Hookean spring, that represents the Young's modulus, and a Newtonian dashpot that represents the viscosity. A material that exhibit properties described in this model is a Maxwell material. Another physical model used is called the Kelvin-Voigt Model and a material that follow this model is called a Kelvin–Voigt material.[44] In order to describe the time-dependent creep and stress-relaxation behavior of hydrogel, a variety of physical lumped parameter models can be used.[40] These modeling methods vary greatly and are extremely complex, so the empirical Prony Series description is commonly used to describe the viscoelastic behavior in hydrogels.[40]
In order to measure the time-dependent viscoelastic behavior of polymers dynamic mechanical analysis is often performed. Typically, in these measurements the one side of the hydrogel is subjected to a sinusoidal load in shear mode while the applied stress is measured with a stress transducer and the change in sample length is measured with a strain transducer.[43] One notation used to model the sinusoidal response to the periodic stress or strain is:
in which G' is the real (elastic or storage) modulus, G" is the imaginary (viscous or loss) modulus.
Poroelasticity
Poroelasticity is a characteristic of materials related to the migration of solvent through a porous material and the concurrent deformation that occurs.[40] Poroelasticity in hydrated materials such as hydrogels occurs due to friction between the polymer and water as the water moves through the porous matrix upon compression. This causes a decrease in water pressure, which adds additional stress upon compression. Similar to viscoelasticity, this behavior is time dependent, thus poroelasticity is dependent on compression rate: a hydrogel shows softness upon slow compression, but fast compression makes the hydrogel stiffer. This phenomenon is due to the friction between the water and the porous matrix is proportional to the flow of water, which in turn is dependent on compression rate. Thus, a common way to measure poroelasticity is to do compression tests at varying compression rates.[45] Pore size is an important factor in influencing poroelasticity. The Kozeny–Carman equation has been used to predict pore size by relating the pressure drop to the difference in stress between two compression rates.[45]
Poroelasticity is described by several coupled equations, thus there are few mechanical tests that relate directly to the poroelastic behavior of the material, thus more complicated tests such as indentation testing, numerical or computational models are utilized. Numerical or computational methods attempt to simulate the three dimensional permeability of the hydrogel network.
Environmental response
The most commonly seen environmental sensitivity in hydrogels is a response to temperature.[46] Many polymers/hydrogels exhibit a temperature dependent phase transition, which can be classified as either an upper critical solution temperature (UCST) or lower critical solution temperature (LCST). UCST polymers increase in their water-solubility at higher temperatures, which lead to UCST hydrogels transitioning from a gel (solid) to a solution (liquid) as the temperature is increased (similar to the melting point behavior of pure materials). This phenomenon also causes UCST hydrogels to expand (increase their swell ratio) as temperature increases while they are below their UCST.[46] However, polymers with LCSTs display an inverse (or negative) temperature-dependence, where their water-solubility decreases at higher temperatures. LCST hydrogels transition from a liquid solution to a solid gel as the temperature is increased, and they also shrink (decrease their swell ratio) as the temperature increases while they are above their LCST.[46]
Applications can dictate for diverse thermal responses. For example, in the biomedical field, LCST hydrogels are being investigated as drug delivery systems due to being injectable (liquid) at room temp and then solidifying into a rigid gel upon exposure to the higher temperatures of the human body.[46] There are many other stimuli that hydrogels can be responsive to, including: pH, glucose, electrical signals, light, pressure, ions, antigens, and more.[46]
Additives
The mechanical properties of hydrogels can be fine-tuned in many ways beginning with attention to their hydrophobic properties.[46][47] Another method of modifying the strength or elasticity of hydrogels is to graft or surface coat them onto a stronger/stiffer support, or by making superporous hydrogel (SPH) composites, in which a cross-linkable matrix swelling additive is added.[7] Other additives, such as nanoparticles and microparticles, have been shown to significantly modify the stiffness and gelation temperature of certain hydrogels used in biomedical applications.[48][49][50]
Processing techniques
While a hydrogel's mechanical properties can be tuned and modified through crosslink concentration and additives, these properties can also be enhanced or optimized for various applications through specific processing techniques. These techniques include electro-spinning, 3D/4D printing, self-assembly, and freeze-casting. One unique processing technique is through the formation of multi-layered hydrogels to create a spatially-varying matrix composition and by extension, mechanical properties. This can be done by polymerizing the hydrogel matrixes in a layer by layer fashion via UV polymerization. This technique can be useful in creating hydrogels that mimic articular cartilage, enabling a material with three separate zones of distinct mechanical properties.[51]
Another emerging technique to optimize hydrogel mechanical properties is by taking advantage of the Hofmeister series. Due to this phenomenon, through the addition of salt solution, the polymer chains of a hydrogel aggregate and crystallize, which increases the toughness of the hydrogel. This method, called "salting out", has been applied to poly(vinyl alcohol) hydrogels by adding a sodium sulfate salt solution.[52] Some of these processing techniques can be used synergistically with each other to yield optimal mechanical properties. Directional freezing or freeze-casting is another method in which a directional temperature gradient is applied to the hydrogel is another way to form materials with anisotropic mechanical properties. Utilizing both the freeze-casting and salting-out processing techniques on poly(vinyl alcohol) hydrogels to induce hierarchical morphologies and anisotropic mechanical properties.[53] Directional freezing of the hydrogels helps to align and coalesce the polymer chains, creating anisotropic array honeycomb tube-like structures while salting out the hydrogel yielded out a nano-fibril network on the surface of these honeycomb tube-like structures. While maintaining a water content of over 70%, these hydrogels' toughness values are well above those of water-free polymers such as polydimethylsiloxane (PDMS), Kevlar, and synthetic rubber. The values also surpass the toughness of natural tendon and spider silk.[53]
Applications
Soft contact lenses
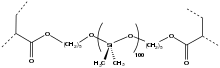
The dominant material for contact lenses are acrylate-
Research


- Coatings for gas evolution reaction electrodes for efficient bubble detachment [55][56][57]
- Breast implants
- Contact lenses (silicone hydrogels, polyacrylamides, polymacon)
- Water sustainability: Hydrogels have emerged as promising materials platforms for solar-powered water purification,[58] water disinfection,[59] and Atmospheric water generator.[60]
- Disposable sanitary napkins[25]
- Dressings for healing of Wound gelsare excellent for helping to create or maintain a moist environment.
- polyethylene oxide, polyAMPS and polyvinylpyrrolidone)
- Encapsulation of quantum dots
- Environmentally sensitive hydrogels (also known as 'smart gels' or 'intelligent gels'). These hydrogels have the ability to sense changes of pH, temperature, or the concentration of metabolite and release their load as result of such a change.[61][62][63]
- Fibers
- Glue
- Granules for holding soil moisture in arid areas
- Air bubble-repellent (superaerophobicity). Can improve the performance and stability of electrodes for water electrolysis.[64]
- Culturing cells: Hydrogel-coated wells have been used for cell culture.[65]
- Cell carrier: Injectable hydrogels can be used to carry drugs or cells for applications in tissue regeneration or 3D bioprinting.[68][69][70] Hydrogels with reversible chemistry are required to allow for fluidization during injection/printing followed by self-healing of the original hydrogel structure.[71]
- Investigate cell biomechanical functions combined with holotomography microscopy
- Provide absorption, desloughing and debriding of necrotic and fibrotic tissue
- hyaluronan, elastin-like polypeptides, and other naturally derived polymers.
- Sustained-release drug delivery systems. Ionic strength, pH and temperature can be used as a triggering factor to control the release of the drug.[73]
- The swelling behavior exhibited by charged hydrogels can be used as a valuable tool for investigating interactions between charged polymers and various species, including multivalent ions, peptides, and proteins.[74] This response arises due to fluctuating osmotic swelling forces resulting from the exchange of counterions within the gel matrix. Particularly significant is its application in assessing the binding of peptide drugs to biopolymers within the body, as the swelling response of the gel can provide insights into these interactions.[75][76]
- Window coating/replacement: Hydrogels are under consideration for reducing infrared light absorption by 75%.[77] Another approach reduced interior temperature using a temperature-responsive hydrogel.[78]
- Thermodynamic electricity generation: When combined with ions allows for heat dissipation for electronic devices and batteries and converting the heat exchange to an electrical charge.[79]
- Water gel explosives
- Controlled release of agrochemicals (pesticides and fertilizer)
- Talin Shock Absorbing Materials - protein-based hydrogels that can absorb supersonic impacts[80]
Biomaterials
Implanted or injected hydrogels have the potential to support tissue regeneration by mechanical tissue support, localized drug or cell delivery,[2] local cell recruitement or immunomodulation, or encapsulation of nanoparticles for local photothermal or brachytherapy.[71] Polymeric drug delivery systems have overcome challenge due to their biodegradability, biocompatibility, and anti-toxicity.[81][82] Materials such as collagen, chitosan, cellulose, and poly (lactic-co-glycolic acid) have been implemented extensively for drug delivery to organs such as eye,[83] nose, kidneys,[84] lungs,[85] intestines,[86] skin[87] and brain.[2] Future work is focused on reducing toxicity, improving biocompatibility, expanding assembly techniques[88]
Hydrogels have been considered as vehicles for drug delivery.[89][68][69][70] They can also be made to mimic animal mucosal tissues to be used for testing mucoadhesive properties.[90][91] They have been examined for use as reservoirs in topical drug delivery; particularly ionic drugs, delivered by iontophoresis.
References
![]() This article incorporates text by Jessica Hutchinson available under the CC BY 3.0 license.
This article incorporates text by Jessica Hutchinson available under the CC BY 3.0 license.
- S2CID 4211987.
- ^ S2CID 264944892.
- ISBN 978-0-323-91248-8, retrieved 2023-01-16
- ISBN 9780081021941.
- ISBN 978-0-08-087862-1.
- ^ ISBN 978-0471238966.
- ^ PMID 25750745.
- S2CID 197928622.
- ISBN 978-3-319-76573-0, retrieved 2023-01-17
- S2CID 229694027.
- PMID 32567846.
- S2CID 135464452.
- ^ PMID 29214058.
- PMID 11755705.
- S2CID 213116098.
- PMID 10517518.
- ISSN 2052-1537.
- PMID 23609001.
- S2CID 136085690.
- doi:10.23939/chcht04.04.297.)
{{cite journal}}: CS1 maint: multiple names: authors list (link) CS1 maint: numeric names: authors list (link - ISBN 978-1-84973-561-2.
- PMID 26240838.
- PMID 19543314.
- ^ PMID 30730212.
- ^ .
- S2CID 245576810.
- PMID 16881042.
- ISSN 2159-6867.
- PMID 23958781.
- S2CID 136880479.
- PMID 19705843.
- ISSN 1744-683X.
- ^ PMID 19921840.
- ^ ISSN 1744-683X.
- PMID 23403581.
- PMID 22955637.
- ISSN 2041-6520.
- PMID 20131781.
- PMID 21761057.
- ^ S2CID 136844625.
- ISBN 978-0-12-812278-5.
- PMID 23946054.
- ^ PMID 8866026.
- ^ Roylance D. ""Engineering viscoelasticity"" (PDF). Modules in Mechanics of Materials. Massachusetts Institute of Technology. Retrieved 11 May 2021.
- ^ S2CID 103246330.
- ^ PMID 11744175.
- ISSN 1742-6588.
- PMID 30986948.
- S2CID 205236639.
- PMID 30380606.
- PMID 21723599.
- S2CID 227258845.
- ^ S2CID 232048202.
- ISBN 9780471484943.
- PMID 32300656.
- S2CID 249355500.
- S2CID 254387206.
- S2CID 211036014.
- S2CID 236174198.
- PMID 35589809.
- PMID 26374941.
- PMID 31355332.
- PMID 35409025.
- PMID 32300656.
- S2CID 9036803.
- ISBN 978-1-78262-242-0.
- PMID 25211200.
- ^ PMID 30275970.
- ^ PMID 28584674.
- ^ S2CID 199574808.
- ^ PMID 35930422.
- ISSN 2046-2069.
- ISSN 1359-0294.
- PMID 16375370.
- PMID 36135299.
- PMID 35500690.
- ^ Irving, Michael (2022-08-31). "Hydrogel glass windows let in more light and less heat". New Atlas. Retrieved 2022-09-26.
- . Retrieved 15 July 2022.
- ^ "A new way to cool down electronic devices, recover waste heat". Phys.org. April 22, 2020. Retrieved April 23, 2020.
- ^ Lavars, Nick (2022-12-15). "New protein-based armor material can withstand supersonic impacts". New Atlas. Retrieved 2022-12-25.
- S2CID 24843309.
- PMID 22192467.
- PMID 23376126.
- PMID 22361096.
- PMID 10320229.
- S2CID 31364133.
- ISBN 978-3-642-24061-4
- ISSN 1884-4057.
- PMID 19216632.
- PMID 26221632.
- PMID 26440734.
Further reading
- Warren DS, Sutherland SP, Kao JY, et al. (2017). "The Preparation and Simple Analysis of a Clay Nanoparticle Composite Hydrogel". Journal of Chemical Education. 94 (11): 1772–1779. ISSN 0021-9584.