Paracoccidioidomycosis
| Paracoccidioidomycosis | |
|---|---|
| Other names | South American blastomycosis, Antifungal medication[6] |
| Medication | Itraconazole, amphotericin B,[6] trimethoprim/sulfamethoxazole[7] |
| Deaths | 200 deaths per year in Brazil[1] |
Paracoccidioidomycosis (PCM), also known as South American blastomycosis, is a
The cause is fungi in the genus Paracoccidioides, including Paracoccidioides brasiliensis and Paracoccidioides lutzii,[8] acquired by breathing in fungal spores.[6]
Diagnosis is by sampling of
It is endemic to Central and South America,[9] and is considered a type of neglected tropical disease.[8] In Brazil, the disease causes around 200 deaths per year.[1]
Signs and symptoms
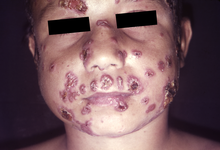
Asymptomatic lung infection is common, with fewer than 5% of infected individuals developing clinical disease.[10]
It can occur as a mouth and skin type, lymphangitic type, multi-organ involvement type (particularly lungs), or mixed type.[1][6] If there are mouth ulcers or skin lesions, the disease is likely to be widespread.[1] There may be no symptoms, or it may present with fever, sepsis, weight loss, large glands, or a large liver and spleen.[4][7]
Two presentations are known, firstly the
Juvenile (acute/subacute) form
The juvenile, acute form is characterised by symptoms, such as fever, weight loss and
Adult (chronic) form
The chronic form presents months to years after the initial infection occurs and most frequently presents with dry cough and shortness of breath.
Up to 70% of cases have mucosal involvement, with lesions often found in the mouth,
Cause
Paracoccidioidomycosis is caused by two species of
Human to human transmission has never been proven.[21]
Mechanism
Primary infection, although poorly understood due to lack of data, is thought to occur through inhalation of the
After inhalation into the alveoli, there is rapid multiplication of the organism in the lung tissue, sometimes spreading via the venous and lymphatic systems.[14] Approximately 2% of people develop clinical features after the initial asymptomatic infection.[15]
The type of immune response determines the clinical manifestation of the infection, with children and
Lung involvement subsequently occurs after a dormant phase, manifesting in upper respiratory tract symptoms, and lung infiltrates on imaging.[15] The commonest, chronic form, is almost certainly a reactivation of the disease,[15] and may develop into progressive scarring of the lungs (pulmonary fibrosis).[22]
It can cause disease in those with normal immune function, although immunosuppression increases the aggressiveness of the fungus. It rarely causes disease in fertile-age women, probably due to a protective effect of estradiol.[23]
Diagnosis
More than 90% of cases can be diagnoses with direct
In the juvenile form, lung abnormalities are shown in high-resolution
Culture of P. brasiliensis takes between 20 and 30 days, requiring multiple samples and culture media. Initial culture can occur at room temperature, however after growth is noted, confirmation occurs by incubating at to 36-37 degrees to transform the fungus into yeast cells.[14]
Differential diagnosis
The disease can appear similar to
Treatment
Both P. brasiliensis and P. lutzii are
Epidemiology
Paracoccidioidomycosis is endemic in rural areas of Latin America, from southern Mexico to Argentina, and is also found in Brazil, Colombia, Venezuela, Ecuador and Paraguay.[13][15] An epidemic outbreak has never been observed.[13] It has the highest prevalence of all systemic mycoses (fungal infections) in the area.[12] As many as 75% of people in endemic areas have been estimated to be infected with the asymptomatic form (up to 10 million people), with 2% developing clinically significant disease.[12] Morbidity and mortality is strongly associated with patient's socioeconomic background,[12] with most adult patients being male agricultural workers.[26] Other risk factors include smoking, alcohol use, HIV co-infection or other immunosuppression.[21] 80% of reported cases are in Brazil, in the southeast, midwest, and south, spreading in the 1990s to the Amazon area. Most of the remaining infections are in Argentina, Colombia and Venezuela.[21] Most epidemiological reports have focused on P. brasliensis, with P. lutzii epidemiology poorly understood as of 2015.[21]
Rising cases have been linked to agriculturalization and deforestation in Brazil, urbanisation to peripheral city areas with poor infrastructure, as well as increased soil and air humidity.[13][21] One Brazilian indigenous tribe, the Surui, after changing from subsistence agriculture to coffee farming showed higher infection rates than surrounding tribes.[21]
There have also been reports in non-endemic areas with the rise of eco-tourism, in the United States, Europe and Japan.[15] All reported cases were returned travellers from endemic regions.[21]
History
Lutz-Splendore-de Almeida disease[3] is named for the physicians Adolfo Lutz,[27] Alfonso Splendor (1871–1953), an Italo-Brazilian parasitologist[28] and Floriano Paulo de Almeida (1898–1977), a Brazilian pathologist specializing in Pathologic Mycology (Study of Infectious Fungi),[29][30] who first characterized the disease in Brazil in the early 20th century.
See also
- North American blastomycosis
References
- ^ ISBN 978-0-323-54753-6.
- ISBN 978-1-4160-2999-1
- ^ Who Named It?
- ^ a b c d e f g h i "ICD-11 - ICD-11 for Mortality and Morbidity Statistics". icd.who.int. Retrieved 26 June 2021.
- ISBN 978-0-7020-6830-0.
- ^ ISBN 978-0-7020-7870-5.
- ^ ISBN 978-0-323-56866-1.
- ^ PMID 28774696.(subscription required)
- PMID 24173174.
- ^ S2CID 260319905.
- PMID 8472249.
- ^ S2CID 41184245.
- ^ PMID 28746570.
- ^ S2CID 243500706
- ^ PMID 23068148.
- OCLC 28421361.
- ^ PMID 19879504.
- ISBN 9780443068393
- S2CID 84410217.
- PMID 25357210.
- ^ PMID 26465364.
- )
- PMID 17655417.
- PMID 26465367.
- PMID 16625617.
- ISBN 9781588298225
- ^ Lutz A (1908), "Uma mycose pseudococcidioidica localizada no boca e observada no Brasil. Contribuicao ao conhecimento das hypoblastomycoses americanas.", Imprensa Médica (in Portuguese), 16, Rio de Janeiro: 151–163
- ^ Splendore A (1912), "Zimonematosi con localizzazione nella cavita della bocca osservata nel Brasile", Bulletin de la Société de pathologie exotique (in French), 5, Paris: 313–319
- ^ De Almeida FP (1928), "Lesoes cutaneas da blastomicose en cabaios experimentalmente infeetados", Anais da Faculdade de Medicina de Universidade de São Paulo (in Portuguese), 3: 59–64
- ^ de Almeida FP, da Silva Lacaz C (1942), "Micoses broco-pulmonares", Comp. Melhoramentos (in Portuguese), São Paulo: 98 pages
