Parkinson's disease
| Parkinson's disease | |
|---|---|
| Other names | Parkinson disease, idiopathic or primary parkinsonism, hypokinetic rigid syndrome, paralysis agitans, shaking palsy |
| Diagnostic method | Based on symptoms[1] |
| Differential diagnosis | Dementia with Lewy bodies, progressive supranuclear palsy, essential tremor, antipsychotic use,[5] fragile X-associated tremor/ataxia syndrome, Huntington's disease, dopamine-responsive dystonia, Wilson's disease[6] |
| Treatment | Medications, surgery[1] |
| Medication | L-DOPA, dopamine agonists[2] |
| Frequency | 6.2 million (2015)[7] |
| Deaths | 117,400 (2015)[8] |
| Named after | James Parkinson |
Parkinson's disease (PD), or simply Parkinson's, is a long-term
may arise in advanced stages.Most of the cases of Parkinson's disease are
mechanisms as well as medications, lifestyle or previous conditions.Diagnosis is mainly based on
No cure is known, and treatment aims to mitigate symptoms. Initial treatment typically includes L-DOPA, MAO-B inhibitors, or dopamine agonists. As the disease progresses, these medications become less effective and produce a side effect marked by involuntary muscle movements. Diet and certain forms of rehabilitation have shown some effectiveness at improving symptoms. Surgery to place microelectrodes for deep brain stimulation has been used to reduce severe motor symptoms where drugs are ineffective. Evidence for treatments for the non-movement-related symptoms of PD, such as sleep disturbances and emotional problems, is less strong.
The disease is named after English doctor
Classification
A progressive
Parkinson's can be classified in other ways. The
Signs and symptoms
The most recognizable symptoms are movement (motor) related and include tremor,
Motor
Four motor symptoms are considered as cardinal signs in PD: tremor, slowness of movement (bradykinesia), rigidity, and postural instability.[15]
The most common presenting sign is a coarse, slow tremor of the hand at rest, which disappears during voluntary movement of the affected arm and in the deeper stages of sleep.[15] It typically appears in only one hand, eventually affecting both hands as the disease progresses.[15] Frequency of PD tremor is between 4–6 hertz (cycles per second). A common characteristic of tremor is pill-rolling, the tendency of the index finger and thumb to touch and perform together with a circular movement.[15][18] The term derives from the similarity between the movement of people with PD and the early pharmaceutical technique of manually making pills.[18]
Bradykinesia is due to disturbances in motor planning of movement initiation, and associated with difficulties along the whole course of the movement process, from planning to initiation to execution of a movement. Performance of sequential and simultaneous movement is impaired. Bradykinesia is the most handicapping symptom of Parkinson's disease, presenting as difficulties with everyday tasks such as dressing, feeding, and bathing. It leads to particular difficulty in carrying out two independent motor activities at the same time, and can be made worse by emotional stress or concurrent illnesses. Paradoxically, people with PD can ride a bicycle or climb stairs more easily than walk on the level. Although most physicians may readily notice bradykinesia, formal assessment requires persons to do repetitive movements with their fingers and feet.[19]
In parkinsonism, rigidity or hypokinesia can be uniform, known as lead-pipe rigidity, or ratcheted, known as cogwheel rigidity.[11][15] The combination of tremor and increased tone is considered to be at the origin of cogwheel rigidity.[20] Rigidity may be associated with joint pain; such pain being a frequent initial manifestation of the disease.[15] In early stages of PD, rigidity is asymmetrical and tends to affect the neck and shoulder muscles before the muscles of the face and extremities.[21] With the progression of the disease, rigidity typically affects the whole body and reduces the ability to move.
Postural instability is typical in the later stages of the disease, leading to impaired balance and frequent falls,[22] and secondarily to bone fractures, loss of confidence, and reduced mobility.[23] Instability is absent in the initial stages, especially in younger people, especially before the development of bilateral symptoms.[24] Up to 40% of people diagnosed with PD may experience falls, and around 10% may have falls weekly, with the number of falls being related to the severity of PD.[15]
Other recognized motor signs and symptoms include gait and posture disturbances such as
Cognitive
PD causes neuropsychiatric disturbances ranging from mild to severe including disorders of cognition, mood, behavior, and thought.[15] Cognitive disturbances can occur in the early stages or before diagnosis, and increase in prevalence with duration of the disease.[15][27] The most common cognitive deficit is executive dysfunction, which can include problems with planning, cognitive flexibility, abstract thinking, rule acquisition, inhibiting inappropriate actions, initiating appropriate actions, working memory, and control of attention.[27][28] Other cognitive difficulties include slowed cognitive processing speed, impaired recall, and impaired perception and estimation of time.[27][28] Nevertheless, improvement appears when recall is aided by cues.[27] Visuospatial difficulties are a part of the disease, seen for example when the individual is asked to perform tests of facial recognition and perception of the orientation of drawn lines.[27][28]
Psychosis
Neuropsychiatric
Behavior and mood alterations are more common in PD without cognitive impairment than in the general population and are usually present in PD with dementia. The most frequent mood difficulties are depression, apathy, and anxiety.[15] Depression impacts an estimated 20% to 35% of patients, and may appear at any stage of the disease. It can manifest with symptoms common to the disease process (fatigue, insomnia, and difficulty with concentration), which makes diagnosis difficult. The imbalance and changes in dopamine, serotonin, and noradrenergic hormones and functional impairment are causes of depression in PD-affected people.[29][33] Suicidal ideation is higher than in the general population, but suicidal attempts themselves are lower.[29][33] Risk factors for depression include disease onset under age 50, being female, previous history of depression, or severe motor symptoms.[29]
Apathy and anhedonia are defined as a loss of motivation and an impaired ability to experience pleasure[36] and are symptoms classically associated with depression, but differ in PD-affected people in treatment and mechanism. Apathy presents in around 16.5–40%. Symptoms of apathy include reduced initiative/interests in new activities or the world around them, emotional indifference, and loss of affection or concern for others.[29] Apathy is associated with deficits in cognitive functions including executive and verbal memory.[33] Anhedonia occurs in 5–75% of people with PD, depending on the study population assessed and overlap with apathy.[37]
Impulse-control disorders, including pathological gambling, compulsive sexual behavior, binge eating, compulsive shopping, and reckless generosity, can be medication-related, particularly orally active dopamine agonists. The dopamine dysregulation syndrome – with wanting of medication contributing to overuse – is a rare complication of levodopa use.[38]
Gastrointestinal
Gastrointestinal issues in Parkinson's disease include
Individuals with Parkinson's have alpha-synuclein deposits in the digestive tract as well as the brain.[41] Constipation is one of the symptoms associated with an increased risk of PD and may precede diagnosis of PD.[41]
Other
Sleep disorders occur with PD and can be worsened by medications.[15] Symptoms can manifest as daytime drowsiness (including sudden sleep attacks resembling narcolepsy), disturbances in rapid eye movement sleep, or insomnia.[15] REM behavior disorder may begin years before the development of motor or cognitive elements of PD or dementia with Lewy bodies.[42]
Alterations in the autonomic nervous system can lead to
Changes in perception may include an impaired sense of smell, disturbed vision, pain, and paresthesia (tingling and numbness).[15] These symptoms can occur years before diagnosis of the disease.[15]
Causes and risk factors
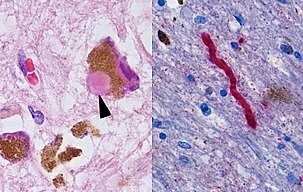
The typical motor symptoms of Parkinson's are due to the deaths of dopaminergic neurons in the substantia nigra pars compacta (SNpc) brain region, resulting in a dopamine deficit in the striatum. Surviving neurons usually possess Lewy bodies—heavily composed of alpha-synuclein—which disrupt cellular processes.[43]
However, the underlying cause of Parkinson's is unknown,[44] and none of the proposed risk factors have been conclusively proven.[45] Age is the most significant risk factor.[46] The most frequently replicated environmental relationships are an increased risk in those exposed to pesticides and a reduced risk in smokers.[45][47] Research indicates that PD results from a complex interaction between genetic and environmental factors.[4] Only a minority of cases can be attributed solely to genetic causes. As idiopathic PD can last and progress over decades, the timing of exposure factor may influence the progression or severity of certain stages.[48]
Genetic
Research indicates that PD results from a complex interaction between genetic and
As of 2022, around 90 genetic risk loci have been identified.
Alpha-synuclein, a protein encoded by SNCA gene mutations, is the main component of the
Non-genetic
Although the vast majority or Parkinson's cases are believed to be non-genetic, identifying environmental risk factors and causality is extremely difficult due to the disease's often decade-long prodromal period.[48] However, multi-decade studies have identified an increased likelihood of Parkinson's in association with agricultural work, pesticide exposure, and rural habitation.[58] In particular, exposure to pesticides like paraquat, rotenone, benomyl, and mancozeb are associated with the onset of Parkinson's disease.[59][60] The neurotoxic mechanisms for these pesticides have largely been identified: inhibition of the mitochondrial electron transport chain, increased oxidative stress, or inhibition of enzymes like aldehyde dehydrogenase and acetylcholinesterase.[61] For most patients, consumption of pesticide-treated food products is believed to be the primary source of exposure.[62]
Some medical drugs are implicated in parkinsonism; drug-induced parkinsonism is normally reversible by stopping the offending agent,[63] such as phenothiazines, butyrophenones, metoclopramide, and Tetrabenazine. MPTP is a drug known for causing irreversible parkinsonism: consequently, it is commonly used in Parkinson's animal-model research.[63][64][65] Low concentrations of urate in the blood are associated with an increased risk.[66] Moreover, a possible link exists between PD and Helicobacter pylori infection that can prevent the absorption of some drugs, including levodopa.[67][68]
Chlorinated solvents, used in commercial and industrial application like dry cleaning and degreasing, are associated with increased PD risk, particularly
Pathophysiology

The main
Five major pathways in the brain connect other brain areas to the basal ganglia. These are known as the motor, oculomotor, associative, limbic, and orbitofrontal circuits. Names indicate the main projection area of each circuit.[78] All are affected in PD, and their disruption causes movement-, attention- and learning-related symptoms of the disease.[78] Scientifically, the motor circuit has been examined the most intensively.[78]
Since 1980, a particular conceptual model of the motor circuit and its alteration with PD has been of influence although some limitations have been pointed out which have led to modifications.[78] In this model, the basal ganglia normally exert a constant inhibitory influence on a wide range of motor systems, preventing them from becoming active at inappropriate times. When a decision is made to perform a particular action, inhibition is reduced for the required motor system, thereby releasing it for activation. Dopamine acts to facilitate this release of inhibition, so high levels of dopamine function tend to promote motor activity, while low levels of dopamine function, such as occur in PD, demand greater exertions of effort for any given movement. The result of dopamine depletion is to produce hypokinesia, an overall reduction in motor output.[78] Drugs that are used to treat PD, conversely, may produce excessive dopamine activity, allowing motor systems to be activated at inappropriate times and thereby producing dyskinesias.[78]
Brain cell death
One mechanism causing brain cell death results from abnormal accumulation of the protein alpha-synuclein bound to
Other mechanisms include proteasomal and lysosomal systems dysfunction and reduced mitochondrial activity.[80] Iron accumulation in the substantia nigra is typically observed in conjunction with the protein inclusions. It may be related to oxidative stress, protein aggregation, and neuronal death, but the mechanisms are obscure.[81]
Neuroimmune interaction
The neuroimmune interaction is heavily implicated in PD pathology. PD and
Activated microglia influence the activation of astrocytes, converting their neuroprotective phenotype to a neurotoxic one. Astrocytes in healthy brains serve to protect neuronal connections. In Parkinson's disease, astrocytes cannot protect the dopaminergic connections in the striatum. Microglia present antigens via MHC-I and MHC-II to T cells. CD4+ T cells, activated by this process, are able to cross the blood brain barrier (BBB) and release more proinflammatory cytokines, like interferon-γ (IFNγ), TNFα, and IL-1β. Mast cell degranulation and subsequent proinflammatory cytokine release is implicated in BBB breakdown in PD. Another immune cell implicated in PD are peripheral monocytes and have been found in the substantia nigra of people with PD. These monocytes can lead to more dopaminergic connection breakdown. In addition, monocytes isolated from people with Parkinson's disease express higher levels of the PD-associated protein, LRRK2, compared with non-PD individuals via vasodilation.[85] In addition, high levels of pro-inflammatory cytokines, such as IL-6, can lead to the production of C-reactive protein by the liver, another protein commonly found in people with PD, that can lead to an increase in peripheral inflammation.[86][87]
Peripheral inflammation can affect the
Diagnosis
Physician's initial assessment is typically based on medical history and neurological examination.[15] They assess motor symptoms (bradykinesia, rest tremors, etc.) using clinical diagnostic criteria. The finding of Lewy bodies in the midbrain on autopsy is usually considered final proof that the person had PD. The clinical course of the illness over time may diverge from PD, requiring that presentation is periodically reviewed to confirm the accuracy of the diagnosis.[15][89]
Multiple causes can occur for parkinsonism or diseases that look similar. Stroke, certain medications, and toxins can cause "secondary parkinsonism" and need to be assessed during visit.[77][89] Parkinson-plus syndromes, such as progressive supranuclear palsy and multiple system atrophy, must be considered and ruled out appropriately to begin a different treatment and disease progression (anti-Parkinson's medications are typically less effective at controlling symptoms in Parkinson-plus syndromes).[15] Faster progression rates, early cognitive dysfunction or postural instability, minimal tremor, or symmetry at onset may indicate a Parkinson-plus disease rather than PD itself.[90]
Medical organizations have created diagnostic criteria to ease and standardize the diagnostic process, especially in the early stages of the disease. The most widely known criteria come from the UK Queen Square Brain Bank for Neurological Disorders and the U.S. National Institute of Neurological Disorders and Stroke. The Queen Square Brain Bank criteria require slowness of movement (bradykinesia) plus either rigidity, resting tremor, or postural instability. Other possible causes of these symptoms need to be ruled out. Finally, three or more of the following supportive symptoms are required during onset or evolution: unilateral onset, tremor at rest, progression in time, asymmetry of motor symptoms, response to levodopa for at least five years, the clinical course of at least ten years and appearance of dyskinesias induced by the intake of excessive levodopa.[91] Assessment of sudomotor function through electrochemical skin conductance can be helpful in diagnosing dysautonomia.[92]
When PD diagnoses are checked by autopsy, movement disorders experts are found on average to be 79.6% accurate at initial assessment and 83.9% accurate after refining diagnoses at follow-up examinations. When clinical diagnoses performed mainly by nonexperts are checked by autopsy, the average accuracy is 73.8%. Overall, 80.6% of PD diagnoses are accurate, and 82.7% of diagnoses using the Brain Bank criteria are accurate.[93]
Imaging
The metabolic activity of dopamine transporters in the basal ganglia can be directly measured with positron emission tomography and single-photon emission computed tomography scans. It has shown high agreement with clinical diagnoses of PD.[99] Reduced dopamine-related activity in the basal ganglia can help exclude drug-induced Parkinsonism. This finding is nonspecific and can be seen with both PD and Parkinson-plus disorders.[94] In the United States, DaTSCANs are only FDA approved to distinguish PD or Parkinsonian syndromes from essential tremor.[100]
Iodine-123-meta-iodobenzylguanidine
Differential diagnosis
Secondary parkinsonism – The multiple causes of parkinsonism can be differentiated through careful history, physical examination, and appropriate imaging.
Other Parkinson-plus syndromes involve tau, rather than alpha-synuclein. These include progressive supranuclear palsy (PSP) and corticobasal syndrome (CBS). PSP predominantly involves rigidity, early falls, bulbar symptoms, and vertical gaze restriction; it can be associated with frontotemporal dementia symptoms. CBS involves asymmetric parkinsonism, dystonia, alien limb, and myoclonic jerking.[103] Presentation timelines and associated symptoms can help differentiate similar movement disorders from idiopathic Parkinson disease.[medical citation needed]
Vascular parkinsonism is the phenomenon of the presence of Parkinson's disease symptoms combined with findings of vascular events (such as a cerebral stroke). The damaging of the dopaminergic pathways is similar in cause for both vascular parkinsonism and idiopathic PD, and so present with similar symptoms. Differentiation can be made with careful bedside examination, history evaluation, and imaging.[104][105]
Parkinson-plus syndrome – Multiple diseases can be considered part of the Parkinson's plus group, including corticobasal syndrome, multiple system atrophy, progressive supranuclear palsy, and dementia with Lewy bodies. Differential diagnosis can be narrowed down with careful history and physical exam (especially focused on the sequential onset of specific symptoms), progression of the disease, and response to treatment.[106][101] Some key symptoms:[63][101]
- non-fluent aphasia
- Dementia with Lewy bodies – levodopa resistance, cognitive predominance before motor symptoms, and fluctuating cognitive symptoms, (visual hallucinations are common in this disease)
- Essential tremor – This can at first look like parkinsonism, but has key differentiators. In essential tremor, the tremor gets worse with action (improves in PD), a lack of other symptoms is common in PD, and normal DatSCAN is seen.[101]
- Multiple system atrophy – levodopa resistance, rapidly progressive, autonomic failure, stridor, present Babinski sign, cerebellar ataxia, and specific MRI findings
- Progressive supranuclear palsy – levodopa resistance, restrictive vertical gaze, specific MRI findings, and early and different postural difficulties
Other conditions that can have similar presentations to PD include:[101][63]
- Arthritis
- Creutzfeldt–Jakob disease
- Depression
- Dystonia
- Fragile X-associated tremor/ataxia syndrome
- Frontotemporal dementia and parkinsonism linked to chromosome 17
- Huntington's disease
- Idiopathic basal ganglia calcification
- Neurodegeneration with brain iron accumulation
- Normal-pressure hydrocephalus
- Obsessional slowness
- Psychogenic parkinsonism
- Wilson's disease
Prevention
Exercise in middle age may reduce the risk of PD later in life.[107] A review of studies regarding how physical activity (exercise) prevents Parkinson's disease noted a 34% reduction in risk amongst individuals who perform moderate to vigorous physical activity. The potential benefits of exercise in individuals with Parkinson's disease are being researched.[108]
Caffeine appears protective with a greater decrease in risk occurring with a larger intake of caffeinated beverages such as coffee.[109]
Antioxidants, such as vitamins C and E, have been proposed to protect against the disease, but results of studies have been contradictory and no positive effect has been shown.[45] The results regarding fat and fatty acids have been contradictory.[45] Use of nonsteroidal anti-inflammatory drugs (NSAIDs) and calcium channel blockers may be protective.[4] A 2010 meta-analysis found that NSAIDs (apart from aspirin), have been associated with at least a 15% (higher in long-term and regular users) reduction in the incidence of the development of PD.[110] As of 2019[update] meta-analyses have failed to confirm this link. Multiple studies have demonstrated a link between the use of ibuprofen and a decreased risk of Parkinson's development.[111]
Management
Despite ongoing research efforts, Parkinson's disease has no known cure. Patients are typically managed by a holistic approach that combines lifestyle modifications with
A 2020 Cochrane review found no certain evidence that cognitive training is beneficial for people with Parkinson's disease, dementia or mild cognitive impairment.[119] The findings are based on low certainty evidence of seven studies.
No standard treatment for PD-associated anxiety exists.[34][page needed]
Medications
This article needs to be updated. The reason given is: PMID 32171387 . (October 2020) |

Levodopa
Levodopa is usually the first drug of choice when treating Parkinson's disease and has been the most widely used PD treatment since the 1980s.[114][120] The motor symptoms of PD are the result of reduced dopamine production in the brain's basal ganglia. Dopamine fails to cross the blood–brain barrier so it cannot be taken as a medicine to boost the brain's depleted levels of dopamine. A precursor of dopamine, levodopa, can pass through to the brain where it is readily converted to dopamine. Administration of levodopa temporarily diminishes the motor symptoms of PD.
Only 5–10% of levodopa crosses the blood–brain barrier. Much of the remainder is metabolized to dopamine elsewhere in the body, causing a variety of side-effects, including nausea, vomiting, and orthostatic hypotension.[121] Carbidopa and benserazide are dopa decarboxylase inhibitors that fail to cross the blood–brain barrier and inhibit the conversion of levodopa to dopamine outside the brain, reducing side-effects and improving the availability of levodopa for passage into the brain. One of these drugs is usually taken along with levodopa and is available combined with levodopa in the same pill.[122]
Prolonged use of levodopa is associated with the development of complications, such as involuntary movements (dyskinesias) and fluctuations in the impact of the medication.[114] When fluctuations occur, a person can cycle through phases with good response to medication and reduced PD symptoms ("on state"), and phases with poor response to medication and increased PD symptoms ("off state").[114][123] Using lower doses of levodopa may reduce the risk and severity of these levodopa-induced complications.[124] A former strategy, called "drug holidays", to reduce levodopa-related dyskinesia and fluctuations was to withdraw levodopa medication for some time,[120] which can bring on dangerous side-effects such as neuroleptic malignant syndrome and is discouraged.[114] Most people with PD eventually need levodopa and later develop levodopa-induced fluctuations and dyskinesias.[114] Adverse effects of levodopa, including dyskinesias, may mistakenly influence people affected by the disease and sometimes their healthcare professionals to delay treatment, which reduces potential for optimal results.[medical citation needed]
Levodopa is available in oral, inhalation, and infusion form; inhaled levodopa can be used when oral levodopa therapy has reached a point where "off" periods have increased in length.[125][126]
COMT inhibitors

During the course of PD, affected people can experience a wearing-off phenomenon, where a recurrence of symptoms occurs after a dose of levodopa, but right before their next dose.[77] Catechol-O-methyltransferase (COMT) is a protein that degrades levodopa before it can cross the blood–brain barrier and COMT inhibitors allow for more levodopa to cross.[128] They are normally used in the management of later symptoms, but can be used in conjunction with levodopa/carbidopa when a person is experiencing the wearing off-phenomenon with their motor symptoms.[77][120]
Three COMT inhibitors are used to treat adults with PD and end-of-dose motor fluctuations – opicapone, entacapone, and tolcapone.[77] Tolcapone has been available for but its usefulness is limited by possible liver damage complications requiring liver-function monitoring.[129][77][128][63] Entacapone and opicapone cause little alteration to liver function.[128][130][131] Licensed preparations of entacapone contain entacapone alone or in combination with carbidopa and levodopa.[132][63][133] Opicapone is a once-daily COMT inhibitor.[134][77]
Dopamine agonists
Dopamine agonists that bind to dopamine receptors in the brain have similar effects to levodopa.[114] These were initially used as a complementary therapy to levodopa for individuals experiencing levodopa complications (on-off fluctuations and dyskinesias); they are mainly used on their own as first therapy for the motor symptoms of PD with the aim of delaying the initiation of levodopa therapy, thus delaying the onset of levodopa's complications.[114][135] Dopamine agonists include bromocriptine, pergolide, pramipexole, ropinirole, piribedil, cabergoline, apomorphine, and lisuride.
Though dopamine agonists are less effective than levodopa at controlling PD motor symptoms, they are effective enough to manage these symptoms in the first years of treatment.[11] Dyskinesias due to dopamine agonists are rare in younger people who have PD, but along with other complications, become more common with older age at onset.[11] Thus, dopamine agonists are the preferred initial treatment for younger-onset PD, and levodopa is preferred for older-onset PD.[11]
Dopamine agonists produce side effects, including drowsiness, hallucinations, insomnia, nausea, and constipation.[114][120] Side effects appear with minimal clinically effective doses giving the physician reason to search for a different drug.[114] Agonists have been related to impulse-control disorders (such as increased sexual activity, eating, gambling, and shopping) more strongly than other antiparkinson medications.[136][120]
Apomorphine, a dopamine agonist, may be used to reduce off periods and dyskinesia in late PD.
MAO-B inhibitors
MAO-B inhibitors (safinamide, selegiline and rasagiline) increase the amount of dopamine in the basal ganglia by inhibiting the activity of monoamine oxidase B, an enzyme that breaks down dopamine.[114] They have been found to help alleviate motor symptoms when used as monotherapy (on their own); when used in conjunction with levodopa, time spent in the off phase is reduced.[138][120] Selegiline has been shown to delay the need for beginning levodopa, suggesting that it might be neuroprotective and slow the progression of the disease.[139] An initial study indicated that selegiline in combination with levodopa increased the risk of death, but this has been refuted.[140]
Common side effects are nausea, dizziness, insomnia, sleepiness, and (in selegiline and rasagiline) orthostatic hypotension.[139][77] MAO-Bs are known to increase serotonin and cause a potentially dangerous condition known as serotonin syndrome.[139]
Other drugs
Other drugs such as amantadine may be useful as treatment of motor symptoms, but evidence for use is lacking.[114][141] Anticholinergics should not be used for dyskinesia or motor fluctuations but may be considered topically for drooling.[120] A diverse range of symptoms beyond those related to motor function can be treated pharmaceutically.[142] Examples are the use of quetiapine or clozapine for psychosis, cholinesterase inhibitors or memantine for dementia, and modafinil for excessive daytime sleepiness.[142][143][120] In 2016, pimavanserin was approved for the management of PD psychosis.[144] Doxepin and rasagline may reduce physical fatigue in PD.[145]
Surgery

Treating motor symptoms with surgery was once a common practice but the discovery of levodopa has decreased the amount of procedures.[146] Studies have led to great improvements in surgical techniques, so surgery can be used in people with advanced PD for whom drug therapy is no longer sufficient.[146] Surgery for PD can be divided in two main groups – lesional and deep brain stimulation (DBS). Target areas for DBS or lesions include the thalamus, globus pallidus, or subthalamic nucleus.[146] DBS involves the implantation of a medical device called a neurostimulator, which sends electrical impulses to specific parts of the brain. DBS is recommended for people who have PD with motor fluctuations and tremor inadequately controlled by medication, or to those who are intolerant to medication lacking severe neuropsychiatric problems.[147] Other less common surgical therapies involve intentional formation of lesions to suppress overactivity of specific subcortical areas. For example, pallidotomy involves surgical destruction of the globus pallidus to control dyskinesia.[146]
Four areas of the brain have been treated with neural stimulators in PD.[148] These are the globus pallidus interna, thalamus, subthalamic nucleus, and pedunculopontine nucleus. DBS of the globus pallidus interna improves motor function, while DBS of the thalamic DBS improves tremor, but has little impact on bradykinesia or rigidity. DBS of the subthalamic nucleus is usually avoided if a history of depression or neurocognitive impairment is present. DBS of the subthalamic nucleus is associated with a reduction in medication. Pedunculopontine nucleus DBS remains experimental at present. Generally, DBS is associated with 30–60% improvement in motor score evaluations.[148]
Rehabilitation
Exercise programs are recommended in people with PD and recent reviews (2023) have demonstrated their efficacy.
In improving flexibility and range of motion for people experiencing rigidity, generalized relaxation techniques such as gentle rocking have been found to decrease excessive muscle tension. Other effective techniques to promote relaxation include slow rotational movements of the extremities and trunk, rhythmic initiation, diaphragmatic breathing, and meditation techniques.[154] As for gait and addressing the challenges associated with the disease such as hypokinesia, shuffling, and decreased arm swing, physiotherapists have a variety of strategies to improve functional mobility and safety. Areas of interest concerning gait during rehabilitation programs focus on improving gait speed, the base of support, stride length, and trunk and arm-swing movement. Strategies include using assistive equipment (pole walking and treadmill walking), verbal cueing (manual, visual, and auditory), exercises (marching and PNF patterns), and altering environments (surfaces, inputs, open vs. closed).[155] Strengthening exercises have shown improvements in strength and motor function for people with primary muscular weakness and weakness related to inactivity with mild to moderate PD, but reports show an interaction between strength and the time the medications were taken. Therefore, people with PD should perform exercises 45 minutes to one hour after medications when they are capable.[156] Deep diaphragmatic breathing exercises are beneficial in improving chest-wall mobility and vital capacity decreased by a forward flexed posture and respiratory dysfunctions in advanced PD.[157] Exercise may improve constipation.[39] Whether exercise reduces physical fatigue in PD remains unclear.[145]
The
Palliative care
The goal of Palliative care is to improve quality of life for both the patient and family by providing relief from the symptoms and stress of illnesses.[161] As Parkinson's is uncurable, treatments focus on slowing decline and improving quality of life and are therefore palliative.[162]
Palliative care should be involved earlier, rather than later, in the disease course.[163][164] Palliative care specialists can help with physical symptoms, emotional factors such as loss of function and jobs, depression, fear, and existential concerns.[163][164][165]
Along with offering emotional support to both the affected person and family, palliative care addresses goals of care. People with PD may have difficult decisions to make as the disease progresses, such as wishes for feeding tube, noninvasive ventilator or tracheostomy, wishes for or against cardiopulmonary resuscitation, and when to use hospice care.[162] Palliative-care team members can help answer questions and guide people with PD on these complex and emotional topics to help them make decisions based on values.[164][166]
Muscles and nerves that control the digestive process may be affected by PD, resulting in constipation and gastroparesis (prolonged emptying of stomach contents).[39] A balanced diet, based on periodical nutritional assessments, is recommended, and should be designed to avoid weight loss or gain and minimize the consequences of gastrointestinal dysfunction.[39] As the disease advances, swallowing difficulties (dysphagia) may appear. Using thickening agents for liquid intake and an upright posture when eating may be useful; both measures reduce the risk of choking. Gastrostomy can be used to deliver food directly into the stomach.[39]
Levodopa and proteins use the same transportation system in the intestine and the blood–brain barrier, thereby competing for access.[39] Taking them together results in reduced effectiveness of the drug.[39] Therefore, when levodopa is introduced, excessive protein consumption is discouraged in favour of a well-balanced Mediterranean diet. In advanced stages, additional intake of low-protein products such as bread or pasta is recommended for similar reasons.[39] To minimize interaction with proteins, levodopa should be taken 30 minutes before meals.[39] At the same time, regimens for PD restrict proteins during breakfast and lunch, allowing protein intake in the evening.[39]
Prognosis
PD invariably progresses with time. A severity rating method known as the Unified Parkinson's disease rating scale (UPDRS) is the most commonly used metric for a clinical study. A modified version known as the MDS-UPDRS is also used. An older scaling method known as the Hoehn and Yahr scale (originally published in 1967), and a similar scale known as the Modified Hoehn and Yahr scale, have been used. The Hoehn and Yahr scale defines five basic stages of progression.
Motor symptoms may advance aggressively in the early stages of the disease and more slowly later. Untreated, individuals are expected to lose independent
No hard correlation between PD progression and PD disability as a whole has been found as of 2023; however, initial PD disability often mainly concerns the motor symptoms, whereas in later stages it is often mainly related to the non-motor symptoms of the disease. Therapies exist that can improve or alleviate these latter symptoms to some extent.[80][167] As the disease advances, disability is more related to motor symptoms that are uncontrollable by medication (such as difficulties with swallowing and dysartria, and gait and balance problems) and to levodopa-induced complications, which appear in up to 50% of individuals after five years of levodopa usage. Finally, after ten years most people with the disease have autonomic disturbances, sleep problems, mood alterations and cognitive decline; these symptoms, especially cognitive decline, greatly increase disability.[80][167]
The life expectancy of people with PD is reduced. Mortality ratios are around twice those of unaffected people. Cognitive decline and dementia, old age at onset, a more advanced disease state, and presence of swallowing problems are all mortality risk factors. A disease pattern mainly characterized by tremor as opposed to rigidity, though, predicts an improved survival. Death from aspiration pneumonia is twice as common in individuals with PD as in the healthy population.[167]
In 2016, PD resulted in about 211,000 deaths globally, an increase of 161% since 1990.[168] The overall death rate increased by 19% to 1.81 per 100,000 people during that time.[168]
A person with PD has two to six times the risk of dementia compared with the general population.
Epidemiology
Parts of this article (those related to this subsection) need to be updated. (November 2023) |
PD is the second most common
China is predicted to have nearly half of the Parkinson's disease population in the world in 2030.[171] By 2040 the number of patients is expected to grow to approximately 14 million people; this growth has been referred to as the Parkinson's pandemic.[172]
The prevalence of dementia increases with age, and to a lesser degree, duration of the disease.[173]
History
Early sources, including an Egyptian papyrus, an ayurvedic medical treatise, the Bible, and Galen's writings, describe symptoms resembling those of PD.[176] After Galen, no references unambiguously related to PD appear until the 17th century.[176] In the 17th and 18th centuries, Franciscus Sylvius, Hieronymus David Gaubius, John Hunter and Auguste François Chomel wrote about elements of the disease.[176][177][178]
Despite that the first systematic description of the disease is attributed to James Parkinson, the disease itself with all four cardinal signs (tremor, bradykinesia, rigor and postural instability) was described in 1690 by Ferenc Pápai Páriz, in a Hungarian medical text over 120 years before the classical description of James Parkinson. However, as it was published in Hungarian language, his book was ignored in the western medical literature. [179] [180] [181] [182]
In 1817, James Parkinson published his essay reporting six people with paralysis agitans.[183] An Essay on the Shaking Palsy described the characteristic resting tremor, abnormal posture and gait, paralysis and diminished muscle strength, and the way that the disease progresses over time.[184][185] Early neurologists who made further additions to the knowledge of the disease include Trousseau, Gowers, Kinnier Wilson and Erb, and Jean-Martin Charcot, whose studies between 1868 and 1881 increased the understanding of the disease.[183] Among other advances, Charcot made the distinction between rigidity, weakness and bradykinesia.[183] He championed the renaming of the disease in honor of James Parkinson.[183]
In 1912, Frederic Lewy described microscopic particles in affected brains, later named Lewy bodies.[183] In 1919, Konstantin Tretiakoff reported that the substantia nigra was the main cerebral structure affected, but this finding was rejected until it was confirmed by further studies published by Rolf Hassler in 1938.[183] The underlying biochemical changes in the brain were identified in the 1950s, due largely to the work of Arvid Carlsson on the neurotransmitter dopamine and Oleh Hornykiewicz on its role on PD.[186] In 1997, alpha-synuclein was found to be the main component of Lewy bodies by Spillantini, Trojanowski, Goedert, and others.[79]
Anticholinergics and surgery (lesioning of the
An illustration of advanced Parkinson's disease by William Richard Gowers was published in 1886 in A Manual of Diseases of the Nervous System, based on 1879 photographs attributed to Albert Londe.[174][175] A new image was created in 2020 to represent diversity in levels of severity.[190][191]
Society and culture
Social impact
For some people with PD, masked facial expressions and difficulty moderating facial expressions of emotion or recognizing other people's facial expressions can impact social well-being.[26] As the condition progresses, tremor, other motor symptoms, difficulty communicating, or issues with mobility may interfere with social engagement, causing individuals with PD to feel isolated.[192] Public perception and awareness of PD symptoms such as shaking, hallucinating, slurring speech, and being off balance is lacking in some countries and can lead to stigma.[192]
Cost
Parts of this article (those related to this subsection) need to be updated. (August 2020) |
The costs of PD to society are high; in 2007, the largest share of direct cost came from inpatient care and nursing homes, while the share coming from medication was substantially lower.[193] Indirect costs are high, due to reduced productivity and the burden on caregivers.[193] In addition to economic costs, PD reduces quality of life of those with the disease and their caregivers.[193]
A study based on 2017 data estimated the US economic PD burden at $51.9 billion, including direct medical costs of $25.4 billion and $26.5 billion in indirect and non-medical costs. The projected total economic burden surpasses $79 billion by 2037. These findings highlight the need for interventions to reduce PD incidence, delay disease progression, and alleviate symptom burden that may reduce the future economic burden of PD.[194]
Advocacy

The birthday of James Parkinson, 11 April, has been designated as World Parkinson's Day.[183] A red tulip was chosen by international organizations as the symbol of the disease in 2005; it represents the 'James Parkinson' tulip cultivar, registered in 1981 by a Dutch horticulturalist.[195]
Advocacy organizations include the