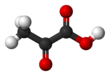
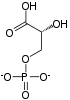
Pyruvic acid
| |||
| Names | |||
|---|---|---|---|
| Preferred IUPAC name
2-Oxopropanoic acid[1] | |||
| Systematic IUPAC name
2-Oxopropionic acid | |||
| Other names
Pyruvic acid[1]
α-Ketopropionic acid Acetylformic acid Pyroracemic acid Acetoic acid Acetylcarboxylic acid Acetocarboxylic acid Oxoacetol | |||
| Identifiers | |||
3D model (
JSmol ) |
|||
| Abbreviations | Pyr | ||
| ChEBI | |||
| ChEMBL | |||
| ChemSpider | |||
| DrugBank | |||
ECHA InfoCard
|
100.004.387 | ||
IUPHAR/BPS |
|||
| KEGG | |||
PubChem CID
|
|||
| UNII | |||
CompTox Dashboard (EPA)
|
|||
| |||
| |||
| Properties | |||
| C3H4O3 | |||
| Molar mass | 88.06 g/mol | ||
| Density | 1.250 g/cm3 | ||
| Melting point | 11.8 °C (53.2 °F; 284.9 K) | ||
| Boiling point | 165 °C (329 °F; 438 K) | ||
| Acidity (pKa) | 2.50[2] | ||
| Related compounds | |||
Other anions
|
|||
Related
keto-acids, carboxylic acids |
|||
Related compounds
|
|||
Except where otherwise noted, data are given for materials in their standard state (at 25 °C [77 °F], 100 kPa).
| |||
Pyruvic acid (
Pyruvic acid can be made from glucose through glycolysis, converted back to carbohydrates (such as glucose) via gluconeogenesis, or converted to fatty acids through a reaction with acetyl-CoA.[3] It can also be used to construct the amino acid alanine and can be converted into ethanol or lactic acid via fermentation.
Pyruvic acid supplies energy to
Chemistry
In 1834, Théophile-Jules Pelouze distilled tartaric acid and isolated glutaric acid and another unknown organic acid. Jöns Jacob Berzelius characterized this other acid the following year and named pyruvic acid because it was distilled using heat.[5][6] The correct molecular structure was deduced by the 1870s.[7]
Pyruvic acid is a colorless liquid with a smell similar to that of
- CH3COCl + KCN → CH3COCN + KCl
- CH3COCN → CH3COCOOH
Biochemistry
This article needs additional citations for verification. (December 2023) |
Pyruvate is an important
These reactions are named after
If insufficient oxygen is available, the acid is broken down
Pyruvate is a key intersection in the network of metabolic pathways. Pyruvate can be converted into carbohydrates via gluconeogenesis, to fatty acids or energy through acetyl-CoA, to the amino acid alanine, and to ethanol. Therefore, it unites several key metabolic processes.[citation needed]

Pyruvic acid production by glycolysis
In the last step of
phosphoenolpyruvate
|
pyruvate kinase | pyruvic acid | |

|

| ||
| ADP | ATP | ||

| |||
| ADP | ATP | ||
PEP carboxykinase
|
|||
Compound C00074 at KEGG Pathway Database. Enzyme 2.7.1.40 at KEGG Pathway Database. Compound C00022 at KEGG Pathway Database.
Click on genes, proteins and metabolites below to link to respective articles.[§ 1]
- ^ The interactive pathway map can be edited at WikiPathways: "GlycolysisGluconeogenesis_WP534".
Decarboxylation to acetyl CoA
Pyruvate decarboxylation by the pyruvate dehydrogenase complex produces acetyl-CoA.
| pyruvate | pyruvate dehydrogenase complex | acetyl-CoA | |

|

| ||
| CoA + NAD+ | CO2 + NADH + H+ | ||
| |||
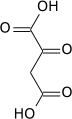
Carboxylation to oxaloacetate
Carboxylation by pyruvate carboxylase produces oxaloacetate.
| pyruvate | pyruvate carboxylase | oxaloacetate
| |

|
| ||
| ATP + CO2 | ADP + Pi | ||

| |||
Transamination to alanine
Transamination by alanine transaminase produces alanine.
| pyruvate | alanine transaminase | alanine | |

|

| ||
glutamate
|
α-ketoglutarate
| ||

| |||
glutamate
|
α-ketoglutarate
| ||
Reduction to lactate
Reduction by lactate dehydrogenase produces lactate.
| pyruvate | lactate dehydrogenase | lactate | |

|

| ||
| NADH | NAD+ | ||

| |||
| NADH | NAD+ | ||
Environmental chemistry
Pyruvic acid is an abundant carboxylic acid in secondary organic aerosols.[12]
Uses
Pyruvate is sold as a weight-loss supplement, though credible science has yet to back this claim. A systematic review of six trials found a statistically significant difference in body weight with pyruvate compared to placebo. However, all of the trials had methodological weaknesses and the magnitude of the effect was small. The review also identified adverse events associated with pyruvate such as diarrhea, bloating, gas, and increase in low-density lipoprotein (LDL) cholesterol. The authors concluded that there was insufficient evidence to support the use of pyruvate for weight loss.[13]
There is also in vitro as well as in vivo evidence in hearts that pyruvate improves metabolism by NADH production stimulation and increases cardiac function.[14][15]
See also
Notes
- ^ ISBN 978-0-85404-182-4.)
{{cite book}}: CS1 maint: DOI inactive as of April 2024 (link - ^ Dawson, R. M. C.; et al. (1959). Data for Biochemical Research. Oxford: Clarendon Press.
- ^ Fox, Stuart Ira (2011). Human Physiology (12th ed.). McGraw=Hill. p. 146.[ISBN missing]
- ^ Ophardt, Charles E. "Pyruvic Acid - Cross Roads Compound". Virtual Chembook. Elmhurst College. Archived from the original on July 31, 2018. Retrieved April 7, 2017.
- ^ Thomson, Thomas (1838). "Chapter II. Of fixed acids Section". Chemistry of organic bodies, vegetables. London: J. B. Baillière. p. 65. Retrieved December 1, 2010.
- .
- .
- ^ "Pyruvic Acid". ChemSpider. Royal Society of Chemistry. Retrieved 21 April 2017.
- ^ Howard, J. W.; Fraser, W. A. "Pyruvic Acid". Organic Syntheses. 4: 63; Collected Volumes, vol. 1, p. 475.
- ^ ISBN 978-0-7167-7108-1.
- ISBN 0-8493-4226-0.
- PMID 34500711.
- S2CID 20241217.
- PMID 26142699.
- S2CID 25126646.
References
- Cody, G. D.; Boctor, N. Z.; Filley, T. R.; Hazen, R. M.; Scott, J. H.; Sharma, A.; Yoder, H. S. Jr (2000). "Primordial Carbonylated Iron-Sulfur Compounds and the Synthesis of Pyruvate". Science. 289 (5483): 1337–1340. S2CID 14911449.