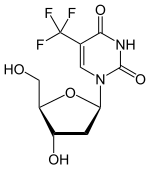
Trifluridine
 | |
| Clinical data | |
|---|---|
| Trade names | Viroptic; Lonsurf (+tipiracil) |
| Other names | α,α,α-trifluorothymidine; 5-trifluromethyl-2′-deoxyuridine; FTD5-trifluoro-2′-deoxythymidine; TFT; CF3dUrd; FTD; F3TDR; F3Thd |
| AHFS/Drugs.com | Monograph |
| License data | |
| Routes of administration | Eye drops; tablets (+tipiracil) |
| ATC code | |
| Legal status | |
| Legal status | |
| Pharmacokinetic data | |
| Bioavailability | Negligible (eye drops); ≥57% (oral) |
| Protein binding | >96% |
| Metabolism | Thymidine phosphorylase |
| Elimination half-life | 12 minutes (eye drops); 1.4–2.1 hrs (combination with tipiracil) |
| Excretion | Mostly via urine |
| Identifiers | |
| |
JSmol) | |
| |
| |
| | |
Trifluridine (also called trifluorothymidine; abbreviation TFT or FTD
Trifluridine was approved for medical use in 1980.[2] It is also a component of the anti-cancer drug trifluridine/tipiracil, which is taken by mouth.
Medical uses
Trifluridine eye drops are used for the treatment of
A
For cancer treatment, the combination trifluridine/tipiracil is used.[citation needed]
Adverse effects
Common side effects of trifluridine eye drops include transient burning, stinging, local irritation, and edema of the eyelids.[3]
Adverse effects of the anti-cancer formulation have only been evaluated for the combination trifluridine/tipiracil, not for the individual components.[citation needed]
Interactions
Only in vitro interaction studies are available. In these, trifluridine used the concentrative nucleoside transporter 1 (CNT1) and equilibrative nucleoside transporters 1 (ENT1) and 2 (ENT2). Drugs that interact with these transporters could influence blood plasma concentrations of trifluridine. Being a thymidine phosphorylase inhibitor, trifluridine could also interact with substrates of this enzyme such as zidovudine.[5]
For the eye drops, trifluridine absorption is negligible,[3] rendering interactions basically irrelevant.
Pharmacology
Mechanism of action (eye drops)
It is a nucleoside analogue, a modified form of deoxyuridine, similar enough to be incorporated into viral DNA replication, but the –CF3 group added to the uracil component blocks base pairing, thus interfering with viral DNA replication.[citation needed]
Pharmacokinetics (eye drops)
Trifluridine passes the cornea and is found in the aqueous humour. Systemic absorption is negligible.[3]
Pharmacokinetics (oral)

Pharmacokinetic data of oral trifluridine have only been evaluated in combination with tipiracil, which significantly affects biotransformation of the former. At least 57% of trifluridine are absorbed from the gut, and highest blood plasma concentrations are reached after two hours in cancer patients. The substance has no tendency to accumulate in the body. Plasma protein binding is over 96%. Trifluridine is metabolised by the enzyme thymidine phosphorylase to 5-trifluoromethyl-2,4(1H,3H)-pyrimidinedione (FTY), and also by glucuronidation. Elimination half-life is 1.4 hours on the first day and increases to 2.1 hours on the twelfth day. It is mainly excreted via the kidneys.[5]
Tipiracil causes Cmax (highest blood plasma concentrations) of trifluridine to increase 22-fold, and its area under the curve 37-fold, by inhibiting thymidine phosphorylase.[5]
Chemistry
The substance is a white crystalline powder. It is freely soluble in
References
- PMID 34406678.
- ISBN 978-1437727029.
- ^ a b c d "Trifluridine". Drugs.com.
- ^ PMID 25879115.
- ^ a b c Haberfeld H, ed. (2015). Austria-Codex (in German). Vienna: Österreichischer Apothekerverlag.
- ^ "Lonsurf Prescribing Information". Drugs.com.
External links
- Costin D, Dogaru M, Popa AS, Cijevschi I (2004). "[Trifluridine therapy in herpetic in keratitis]" [Trifluridine therapy in herpetic in keratitis]. Revista Medico-Chirurgicala a Societatii de Medici Si Naturalisti Din Iasi (in Romanian). 108 (2): 409–412. PMID 15688823.
- Kuster P, Taravella M, Gelinas M, Stepp P (April 1998). "Delivery of trifluridine to human cornea and aqueous using collagen shields". The CLAO Journal. 24 (2): 122–124. PMID 9571274.
- O'Brien WJ, Taylor JL (August 1991). "Therapeutic response of herpes simplex virus-induced corneal edema to trifluridine in combination with immunosuppressive agents". Investigative Ophthalmology & Visual Science. 32 (9): 2455–2461. PMID 1907950.
- "Trifluridine Ophthalmic Solution, 1%" (PDF). Retrieved 2007-03-24.
