Foscarnet
 | |
 | |
| Clinical data | |
|---|---|
| Trade names | Foscavir, Vocarvi, others |
| Other names | phosphonomethanoic acid, dihydroxyphosphinecarboxylic acid oxide |
| AHFS/Drugs.com | Monograph |
| MedlinePlus | a601144 |
| License data | |
| Pregnancy category |
|
| Routes of administration | Intravenous |
| ATC code | |
| Legal status | |
| Legal status | |
| Pharmacokinetic data | |
| Bioavailability | NA |
| Protein binding | 14–17% |
| Elimination half-life | 3.3–6.8 hours |
| Identifiers | |
| |
JSmol) | |
| |
| |
| | |
Foscarnet (phosphonomethanoic acid), known by its brand name Foscavir, is an antiviral medication which is primarily used to treat viral infections involving the
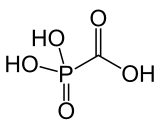
conjugate base of a chemical compound with the formula HO2CPO3H2 (Trisodium phosphonoformate).[5][6]
Foscarnet was approved for medical use in 1991.generic medication.[8]
Medical use
This
CMV retinitis. Foscarnet can be used to treat highly treatment-experienced patients with HIV as part of salvage therapy.[9][10][11]
Mechanism of action
Foscarnet is a structural mimic of the anion pyrophosphate that selectively inhibits the pyrophosphate binding site on viral DNA polymerases at concentrations that do not affect human DNA polymerases.[11]
In individuals treated with the DNA polymerase inhibitors
acyclovir or ganciclovir, HSV or CMV particles can develop mutant protein kinases (thymidine kinase or UL97 protein kinase, respectively) that make them resistant to these antiviral drugs.[12][13] However, unlike acyclovir and ganciclovir, foscarnet is not activated by viral protein kinases, making it useful in acyclovir- or ganciclovir-resistant HSV and CMV infections.[5]
However, acyclovir- or ganciclovir-resistant mutants with alterations in viral DNA polymerase may also be resistant to foscarnet.[14][15]
Administration
Foscarnet is administered by
intravenous infusion or intravitreous injection.[citation needed
]
Side effects
- Nephrotoxicity — increase in serum creatinine levels and renal injury can occur in patients receiving foscarnet.[5][16] Other nephrotoxic drugs should be avoided. Nephrotoxicity is usually reversible and can be reduced by dosage adjustment and adequate hydration.[17]
- Genital ulceration — a less common reported side effect which occurs more in men and usually during induction use of foscarnet.[5] It is most likely a contact dermatitis due to high concentrations of foscarnet in urine. It usually resolves rapidly following discontinuation of the drug.[20]
- CNS — less common side effects of
References
- ^ "Regulatory Decision Summary - Vocarvi". Health Canada. 23 October 2014. Retrieved 4 June 2022.
- ^ "Foscavir- foscarnet sodium injection, solution". DailyMed. 23 April 2020. Retrieved 6 November 2020.
- S2CID 260483894.
- PMID 1706982.
- ^ PMID 32310568. Retrieved 2022-04-04.
- ^ "phosphonoformic acid (CHEBI:127780)". www.ebi.ac.uk. Retrieved 2022-04-04.
- ISBN 978-1437727029.
- ^ "Competitive Generic Therapy Approvals". U.S. Food and Drug Administration (FDA). 29 June 2023. Archived from the original on 29 June 2023. Retrieved 29 June 2023.
- S2CID 24905247.
- S2CID 19856772.
- ^ PMID 17400246.
- S2CID 42775774.
- PMID 15728902.
- S2CID 24584000.
- PMID 16415021.
- S2CID 211537187.
- ^ PMID 1534839.
- S2CID 37250222.
- ^ PMID 32905132.
- PMID 29991980.
Sources
- Dennis L. Kasper, Eugene Braunwald. "Harrison's Manual of Medicine", 16th Edition, Mcgraw-hill, (2005), p. 2244.
External links
- "Foscarnet". Drug Information Portal. U.S. National Library of Medicine.
- "Foscarnet sodium". Drug Information Portal. U.S. National Library of Medicine.
