Allenes

In
History
For many years, allenes were viewed as curiosities but thought to be synthetically useless and difficult to prepare and to work with.
Structure and properties
Geometry

The central carbon atom of allenes forms two
Symmetry

The symmetry and isomerism of allenes has long fascinated organic chemists.
An allene with two different substituents on each of the two carbon atoms will be chiral because there will no longer be any mirror planes. The chirality of these types of allenes was first predicted in 1875 by Jacobus Henricus van 't Hoff, but not proven experimentally until 1935.[9] Where A has a greater priority than B according to the Cahn–Ingold–Prelog priority rules, the configuration of the axial chirality can be determined by considering the substituents on the front atom followed by the back atom when viewed along the allene axis. For the back atom, only the group of higher priority need be considered.
Chiral allenes have been recently used as building blocks in the construction of organic materials with exceptional chiroptical properties.[10] There are a few examples of drug molecule having an allene system in their structure.[11] Mycomycin, an antibiotic with tuberculostatic properties,[12] is a typical example. This drug exhibits enantiomerism due to the presence of a suitably substituted allene system.
Although the semi-localized textbook
Chemical and spectral properties
Allenes differ considerably from other alkenes in terms of their chemical properties. Compared to isolated and conjugated dienes, they are considerably less stable: comparing the isomeric pentadienes, the allenic 1,2-pentadiene has a heat of formation of 33.6 kcal/mol, compared to 18.1 kcal/mol for (E)-1,3-pentadiene and 25.4 kcal/mol for the isolated 1,4-pentadiene.[15]
The C–H bonds of allenes are considerably weaker and more acidic compared to typical vinylic C–H bonds: the bond dissociation energy is 87.7 kcal/mol (compared to 111 kcal/mol in ethylene), while the gas-phase acidity is 381 kcal/mol (compared to 409 kcal/mol for ethylene[16]), making it slightly more acidic than the propargylic C–H bond of propyne (382 kcal/mol).
The 13C NMR spectrum of allenes is characterized by the signal of the sp-hybridized carbon atom, resonating at a characteristic 200-220 ppm. In contrast, the sp2-hybridized carbon atoms resonate around 80 ppm in a region typical for alkyne and nitrile carbon atoms, while the protons of a CH2 group of a terminal allene resonate at around 4.5 ppm — somewhat upfield of a typical vinylic proton.[17]
Allenes possess a rich cycloaddition chemistry, including both [4+2] and [2+2] modes of addition,[18][19] as well as undergoing formal cycloaddition processes catalyzed by transition metals.[20][21] Allenes also serve as substrates for transition metal catalyzed hydrofunctionalization reactions.[22][23][24]
Synthesis
Although allenes often require specialized syntheses, the parent allene,
This mixture, known as MAPP gas, is commercially available. At 298 K, the ΔG° of this reaction is –1.9 kcal/mol, corresponding to Keq = 24.7.[25]
The first allene to be synthesized was penta-2,3-dienedioic acid, which was prepared by Burton and Pechmann in 1887. However, the structure was only correctly identified in 1954.[26]
Laboratory methods for the formation of allenes include:
- from geminal dihalocyclopropanes and organolithium compounds (or metallic sodium or magnesium) in the Skattebøl rearrangement (Doering–LaFlamme allene synthesis) via rearrangement of cyclopropylidene carbenes/carbenoids
- from reaction of certain terminal
- from propargylic halides by SN2′ displacement by an organocuprate[29]
- from dehydrohalogenation of certain dihalides[30]
- from reaction of a triphenylphosphinyl ester with an acid halide, a Wittig reaction accompanied by dehydrohalogenation[31][32]
- from propargylic alcohols via the stereospecificprocess
- from metalation of allene or substituted allenes with BuLi and reaction with electrophiles (RX, R3SiX, D2O, etc.)[33]
The chemistry of allenes has been reviewed in a number of books[2][34][35][36] and journal articles.[3][37][38][39][40][41][42][43][44] Some key approaches towards allenes are outlined in the following scheme:[45][46][47][48]
One of the older methods is the Skattebøl rearrangement
Use and occurrence
Allene itself is the most commonly used member of this family; it exists in equilibrium with propyne as a component of MAPP gas.[58]
Research
The reactivity of substituted allenes has been well explored.[59][60][61][62]
The two π-bonds are located at the 90° angle to each other, and thus require a reagent to approach from somewhat different directions. With an appropriate substitution pattern, allenes exhibit axial chirality as predicted by van’t Hoff as early as 1875.[63][62] Protonation of allenes gives cations 11 that undergo further transformations.[64] Reactions with soft electrophiles (e.g. Br+) deliver positively charged onium ions 13.[65] Transition-metal-catalysed reactions proceed via allylic intermediates 15 and have attracted significant interest in recent years.[66][67] Numerous cycloadditions are also known, including [4+2]-, (2+1)-, and [2+2]-variants, which deliver, e.g., 12, 14, and 16, respectively.[59][68][69][70]
Occurrence
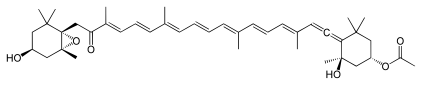
Numerous natural products contain the allene functional group. Noteworthy are the pigments fucoxanthin and peridinin. Little is known about the biosynthesis, although it is conjectured that they are often generated from alkyne precursors.[71]
Allenes serve as ligands in organometallic chemistry. A typical complex is Pt(η2-allene)(PPh3)2. Ni(0) reagents catalyze the cyclooligomerization of allene.[72] Using a suitable catalyst (e.g. Wilkinson's catalyst), it is possible to reduce just one of the double bonds of an allene.[73]
Delta convention
Many rings or ring systems are known by semisystematic names that assume a maximum number of noncumulative bonds. To unambiguously specify derivatives that include cumulated bonds (and hence fewer hydrogen atoms than would be expected from the skeleton), a lowercase delta may be used with a subscript indicating the number of cumulated double bonds from that atom, e.g. 8δ2-benzocyclononene. This may be combined with the λ-convention for specifying nonstandard valency states, e.g. 2λ4δ2,5λ4δ2-thieno[3,4-c]thiophene.[74]
See also
- Compounds with three or more adjacent carbon–carbon double bonds are called cumulenes.
References
![]() This article incorporates text by Oleksandr Zhurakovskyi available under the CC BY 2.5 license.
This article incorporates text by Oleksandr Zhurakovskyi available under the CC BY 2.5 license.
- ^ a b The Chemistry of the Allenes (vol. 1−3); Landor, S. R., Ed.; cademic Press: London, 1982.
- ^ ISSN 0009-2665.
- ^ PMID 14991780.
- .
- ISSN 0368-1769.
- ^ Data from the Web of Science database.
- ISBN 978-0-471-72091-1
- S2CID 4085600.
- PMID 22308109.
- ISSN 0002-7863.
- PMID 14784717.
- hdl:10044/1/41564.
- PMID 29974064.
- doi:10.18434/T4D303. Retrieved 2020-10-17.
- ISBN 978-1-118-90637-8.
- )
- PMID 20111793.
- .
- ISSN 1434-193X.
- PMID 30640446.
- PMID 26890605.
- PMID 29052303.
- PMID 31857913.
- ISSN 0002-7863.
- ISSN 0368-1769.
- ; Collected Volumes, vol. 7, p. 276.
- .
- PMID 22111574.
- ; Collected Volumes, vol. 5, p. 22.
- .
- ; Collected Volumes, vol. 7, p. 232.
- ISSN 0039-7911.
- )
- )
- OCLC 162570992.
- .
- PMID 11749314.
- PMID 19603781.
- PMID 20111793.
- S2CID 189843060.
- PMID 21207554.
- PMID 21391568.
- PMID 21314182.
- ^ PMID 12683779.
- ^ ISSN 0039-7881.
- ^ PMID 21409186.
- ^ PMID 23034723.
- ISSN 0904-213X.
- ISSN 0022-3263.
- PMID 12683778.
- OCLC 850164343.
- PMID 20518460.
- ISSN 0002-7863.
- .
- PMID 15058962.
- PMID 15387613.
- ISBN 978-3527306732.
- ^ .
- PMID 19603781.
- PMID 22271630.
- ^ PMID 16011326.
- ^ Van ’t Hoff, J. H. La Chimie dans l’Espace; P.M. Bazendijk, 1875; p. 43.
- OCLC 607520014.
- OCLC 162570992.
- S2CID 97529095.
- PMID 12108979.
- PMID 23861294.
- .
- ISSN 0022-3263.
- ISBN 9783527619573.
- .
- .
- S2CID 97759274.
Further reading
- Brummond, Kay M. (editor). Allene chemistry (special thematic issue). Beilstein Journal of Organic Chemistry 7: 394–943.





