Limestone
Torcal de Antequera nature reserve of Málaga, Spain | |
| Composition | |
|---|---|
| Calcium carbonate: inorganic crystalline calcite or organic calcareous material |
Limestone (
About 20% to 25% of sedimentary rock is carbonate rock, and most of this is limestone.
Limestone has numerous uses: as a chemical
Description

Limestone is composed mostly of the
Limestone often contains variable amounts of
Limestone is commonly white to gray in color. Limestone that is unusually rich in organic matter can be almost black in color, while traces of
Although limestones show little variability in mineral composition, they show great diversity in texture.[15] However, most limestone consists of sand-sized grains in a carbonate mud matrix. Because limestones are often of biological origin and are usually composed of sediment that is deposited close to where it formed, classification of limestone is usually based on its grain type and mud content.[9]
Grains



Most grains in limestone are skeletal fragments of marine organisms such as
Skeletal grains have a composition reflecting the organisms that produced them and the environment in which they were produced.
Ooids (sometimes called ooliths) are sand-sized grains (less than 2mm in diameter) consisting of one or more layers of calcite or aragonite around a central quartz grain or carbonate mineral fragment. These likely form by direct precipitation of calcium carbonate onto the ooid. Pisoliths are similar to ooids, but they are larger than 2 mm in diameter and tend to be more irregular in shape. Limestone composed mostly of ooids is called an oolite or sometimes an oolitic limestone. Ooids form in high-energy environments, such as the Bahama platform, and oolites typically show crossbedding and other features associated with deposition in strong currents.[20][21]
Oncoliths resemble ooids but show a radial rather than layered internal structure, indicating that they were formed by algae in a normal marine environment.[20]
Peloids are structureless grains of microcrystalline carbonate likely produced by a variety of processes.
Limeclasts are fragments of existing limestone or partially lithified carbonate sediments. Intraclasts are limeclasts that originate close to where they are deposited in limestone, while extraclasts come from outside the depositional area. Intraclasts include grapestone, which is clusters of peloids cemented together by organic material or mineral cement. Extraclasts are uncommon, are usually accompanied by other clastic sediments, and indicate deposition in a tectonically active area or as part of a turbidity current.[26]
Mud
The grains of most limestones are embedded in a matrix of carbonate mud. This is typically the largest fraction of an ancient carbonate rock.[23] Mud consisting of individual crystals less than 5 μm (0.20 mils) in length is described as micrite.[27] In fresh carbonate mud, micrite is mostly small aragonite needles, which may precipitate directly from seawater,[28] be secreted by algae,[29] or be produced by abrasion of carbonate grains in a high-energy environment.[30] This is converted to calcite within a few million years of deposition. Further recrystallization of micrite produces microspar, with grains from 5 to 15 μm (0.20 to 0.59 mils) in diameter.[28]
Limestone often contains larger crystals of calcite, ranging in size from 0.02 to 0.1 mm (0.79 to 3.94 mils), that are described as sparry calcite or sparite. Sparite is distinguished from micrite by a grain size of over 20 μm (0.79 mils) and because sparite stands out under a hand lens or in thin section as white or transparent crystals. Sparite is distinguished from carbonate grains by its lack of internal structure and its characteristic crystal shapes. [31]
Geologists are careful to distinguish between sparite deposited as cement and sparite formed by recrystallization of micrite or carbonate grains. Sparite cement was likely deposited in pore space between grains, suggesting a high-energy depositional environment that removed carbonate mud. Recrystallized sparite is not diagnostic of depositional environment.[31]
Other characteristics
The makeup of a carbonate rock outcrop can be estimated in the field by etching the surface with dilute hydrochloric acid. This etches away the calcite and aragonite, leaving behind any silica or dolomite grains. The latter can be identified by their
Crystals of calcite,
Dense, massive limestone is sometimes described as "marble". For example, the famous
Classification

Folk classification
Robert L. Folk developed a classification system that places primary emphasis on the detailed composition of grains and interstitial material in
Dunham classification
Robert J. Dunham published his system for limestone in 1962. It focuses on the depositional fabric of carbonate rocks. Dunham divides the rocks into four main groups based on relative proportions of coarser clastic particles, based on criteria such as whether the grains were originally in mutual contact, and therefore self-supporting, or whether the rock is characterized by the presence of frame builders and algal mats. Unlike the Folk scheme, Dunham deals with the original porosity of the rock. The Dunham scheme is more useful for hand samples because it is based on texture, not the grains in the sample.[37]
A revised classification was proposed by Wright (1992). It adds some diagenetic patterns to the classification scheme.[38]
Other descriptive terms
Chalk is a soft, earthy, fine-textured limestone composed of the tests of planktonic microorganisms such as foraminifera, while marl is an earthy mixture of carbonates and silicate sediments.[40]
Formation
Limestone forms when calcite or aragonite precipitate out of water containing dissolved calcium, which can take place through both biological and nonbiological processes.[41] The solubility of calcium carbonate (CaCO3) is controlled largely by the amount of dissolved carbon dioxide (CO2) in the water. This is summarized in the reaction:
- CaCO3 + H2O + CO2 → Ca2+ + 2HCO−3
Increases in temperature or decreases in pressure tend to reduce the amount of dissolved CO2 and precipitate CaCO3. Reduction in salinity also reduces the solubility of CaCO3, by several orders of magnitude for fresh water versus seawater. [42]
Near-surface water of the earth's oceans are oversaturated with CaCO3 by a factor of more than six.
The origin of carbonate mud,[30] and the processes by which it is converted to micrite,[45] continue to be a subject of research. Modern carbonate mud is composed mostly of aragonite needles around 5 μm (0.20 mils) in length. Needles of this shape and composition are produced by calcareous algae such as Penicillus, making this a plausible source of mud.[46] Another possibility is direct precipitation from the water. A phenomenon known as whitings occurs in shallow waters, in which white streaks containing dispersed micrite appear on the surface of the water. It is uncertain whether this is freshly precipitated aragonite or simply material stirred up from the bottom, but there is some evidence that whitings are caused by biological precipitation of aragonite as part of a bloom of cyanobacteria or microalgae.[47] However, stable isotope ratios in modern carbonate mud appear to be inconsistent with either of these mechanisms, and abrasion of carbonate grains in high-energy environments has been put forward as a third possibility.[30]
Formation of limestone has likely been dominated by biological processes throughout the Phanerozoic, the last 540 million years of the Earth's history. Limestone may have been deposited by microorganisms in the Precambrian, prior to 540 million years ago, but inorganic processes were probably more important and likely took place in an ocean more highly oversaturated in calcium carbonate than the modern ocean.[48]
Diagenesis
Diagenesis is the process in which sediments are compacted and turned into solid rock. During diagenesis of carbonate sediments, significant chemical and textural changes take place. For example, aragonite is converted to low-magnesium calcite. Diagenesis is the likely origin of pisoliths, concentrically layered particles ranging from 1 to 10 mm (0.039 to 0.394 inches) in diameter found in some limestones. Pisoliths superficially resemble ooids but have no nucleus of foreign matter, fit together tightly, and show other signs that they formed after the original deposition of the sediments.[49]


Silicification occurs early in diagenesis, at low pH and temperature, and contributes to fossil preservation.[50] Silicification takes place through the reaction:[50]
Fossils are often preserved in exquisite detail as chert.[50][51]
Cementing takes place rapidly in carbonate sediments, typically within less than a million years of deposition. Some cementing occurs while the sediments are still under water, forming
As carbonate sediments are increasingly deeply buried under younger sediments, chemical and mechanical compaction of the sediments increases. Chemical compaction takes place by pressure solution of the sediments. This process dissolves minerals from points of contact between grains and redeposits it in pore space, reducing the porosity of the limestone from an initial high value of 40% to 80% to less than 10%.[53] Pressure solution produces distinctive stylolites, irregular surfaces within the limestone at which silica-rich sediments accumulate. These may reflect dissolution and loss of a considerable fraction of the limestone bed. At depths greater than 1 km (0.62 miles), burial cementation completes the lithification process. Burial cementation does not produce stylolites.[54]
When overlying beds are eroded, bringing limestone closer to the surface, the final stage of diagenesis takes place. This produces secondary porosity as some of the cement is dissolved by rainwater infiltrating the beds. This may include the formation of vugs, which are crystal-lined cavities within the limestone.[54]
Diagenesis may include conversion of limestone to dolomite by magnesium-rich fluids. There is considerable evidence of replacement of limestone by dolomite, including sharp replacement boundaries that cut across bedding.[55] The process of dolomitization remains an area of active research,[56] but possible mechanisms include exposure to concentrated brines in hot environments (evaporative reflux) or exposure to diluted seawater in delta or estuary environments (Dorag dolomitization).[57] However, Dorag dolomitization has fallen into disfavor as a mechanism for dolomitization,[58] with one 2004 review paper describing it bluntly as "a myth".[56] Ordinary seawater is capable of converting calcite to dolomite, if the seawater is regularly flushed through the rock, as by the ebb and flow of tides (tidal pumping).[55] Once dolomitization begins, it proceeds rapidly, so that there is very little carbonate rock containing mixed calcite and dolomite. Carbonate rock tends to be either almost all calcite/aragonite or almost all dolomite.[57]
Occurrence
About 20% to 25% of sedimentary rock is carbonate rock,
Most limestone was formed in shallow marine environments, such as

Limestone formations tend to show abrupt changes in thickness. Large moundlike features in a limestone formation are interpreted as ancient reefs, which when they appear in the geologic record are called bioherms. Many are rich in fossils, but most lack any connected organic framework like that seen in modern reefs. The fossil remains are present as separate fragments embedded in ample mud matrix. Much of the sedimentation shows indications of occurring in the intertidal or supratidal zones, suggesting sediments rapidly fill available accommodation space in the shelf or platform.[65] Deposition is also favored on the seaward margin of shelves and platforms, where there is upwelling deep ocean water rich in nutrients that increase organic productivity. Reefs are common here, but when lacking, ooid shoals are found instead. Finer sediments are deposited close to shore.[66]
The lack of deep sea limestones is due in part to rapid subduction of oceanic crust, but is more a result of dissolution of calcium carbonate at depth. The solubility of calcium carbonate increases with pressure and even more with higher concentrations of carbon dioxide, which is produced by decaying organic matter settling into the deep ocean that is not removed by photosynthesis in the dark depths. As a result, there is a fairly sharp transition from water saturated with calcium carbonate to water unsaturated with calcium carbonate, the lysocline, which occurs at the calcite compensation depth of 4,000 to 7,000 m (13,000 to 23,000 feet). Below this depth, foraminifera tests and other skeletal particles rapidly dissolve, and the sediments of the ocean floor abruptly transition from carbonate ooze rich in foraminifera and coccolith remains (Globigerina ooze) to silicic mud lacking carbonates.[67]

In rare cases, turbidites or other silica-rich sediments bury and preserve benthic (deep ocean) carbonate deposits. Ancient benthic limestones are microcrystalline and are identified by their tectonic setting. Fossils typically are foraminifera and coccoliths. No pre-Jurassic benthic limestones are known, probably because carbonate-shelled plankton had not yet evolved.[68]
Limestones also form in freshwater environments.
Limestones may also form in
Limestone and living organisms

Most limestone is formed by the activities of living organisms near reefs, but the organisms responsible for reef formation have changed over geologic time. For example,
Limestone shows the same range of
The
Micritic mud mounds
Micricitic mud mounds are subcircular domes of micritic calcite that lacks internal structure. Modern examples are up to several hundred meters thick and a kilometer across, and have steep slopes (with slope angles of around 50 degrees). They may be composed of peloids swept together by currents and stabilized by
Mud mounds are found throughout the geologic record, and prior to the
Organic reefs
Organic reefs form at low latitudes in shallow water, not more than a few meters deep. They are complex, diverse structures found throughout the fossil record. The frame-building organisms responsible for organic reef formation are characteristic of different geologic time periods:
Organic reefs typically have a complex internal structure. Whole body fossils are usually abundant, but ooids and interclasts are rare within the reef. The core of a reef is typically massive and unbedded, and is surrounded by a talus that is greater in volume than the core. The talus contains abundant intraclasts and is usually either floatstone, with 10% or more of grains over 2mm in size embedded in abundant matrix, or rudstone, which is mostly large grains with sparse matrix. The talus grades to planktonic fine-grained carbonate mud, then noncarbonate mud away from the reef.[81]
Limestone landscape

Karst regions overlying limestone bedrock tend to have fewer visible above-ground sources (ponds and streams), as surface water easily drains downward through joints in the limestone. While draining, water and organic acid from the soil slowly (over thousands or millions of years) enlarges these cracks, dissolving the calcium carbonate and carrying it away in solution. Most cave systems are through limestone bedrock. Cooling groundwater or mixing of different groundwaters will also create conditions suitable for cave formation.[85]
Coastal limestones are often eroded by organisms which bore into the rock by various means. This process is known as
Bands of limestone emerge from the Earth's surface in often spectacular rocky outcrops and islands. Examples include the
The Florida Keys, islands off the south coast of Florida, are composed mainly of oolitic limestone (the Lower Keys) and the carbonate skeletons of coral reefs (the Upper Keys), which thrived in the area during interglacial periods when sea level was higher than at present.[97]
Unique habitats are found on alvars, extremely level expanses of limestone with thin soil mantles. The largest such expanse in Europe is the Stora Alvaret on the island of Öland, Sweden.[98] Another area with large quantities of limestone is the island of Gotland, Sweden.[99] Huge quarries in northwestern Europe, such as those of Mount Saint Peter (Belgium/Netherlands), extend for more than a hundred kilometers.[100]
Uses
Limestone can be processed into many various forms such as brick, cement, powdered/crushed, or as a filler.[102] Limestone is readily available and relatively easy to cut into blocks or more elaborate carving.[101] Ancient American sculptors valued limestone because it was easy to work and good for fine detail. Going back to the Late Preclassic period (by 200–100 BCE), the Maya civilization (Ancient Mexico) created refined sculpture using limestone because of these excellent carving properties. The Maya would decorate the ceilings of their sacred buildings (known as lintels) and cover the walls with carved limestone panels. Carved on these sculptures were political and social stories, and this helped communicate messages of the king to his people.[106] Limestone is long-lasting and stands up well to exposure, which explains why many limestone ruins survive. However, it is very heavy (density 2.6[107]), making it impractical for tall buildings, and relatively expensive as a building material.
Limestone was most popular in the late 19th and early 20th centuries. Railway stations, banks and other structures from that era were made of limestone in some areas. It is used as a
Limestone was also a very popular building block in the Middle Ages in the areas where it occurred, since it is hard, durable, and commonly occurs in easily accessible surface exposures. Many medieval churches and castles in Europe are made of limestone.
-
Limestone quarry atCedar Creek, Virginia, US
-
Limestone as building material
-
Limestone is used worldwide as building material.
Limestone is the raw material for production of lime, primarily known for treating soils, purifying water and

Other uses include:
- It is the raw material for the manufacture of
- Pulverized limestone is used as a soil conditioner to neutralize acidic soils (agricultural lime).[115]
- Is crushed for use as aggregate—the solid base for many roads as well as in asphalt concrete.[59]
- As a reagent in flue-gas desulfurization, where it reacts with sulfur dioxide for air pollution control.[116]
- In glass making, particularly in the manufacture of soda–lime glass.[117]
- As an additive toothpaste, paper, plastics, paint, tiles, and other materials as both white pigment and a cheap filler.[118]
- As rock dust, to suppress methane explosions in underground coal mines.[119]
- Purified, it is added to bread and cereals as a source of calcium.[120]
- As a calcium supplement in livestock feed, such as for poultry (when ground up).[121]
- For remineralizing and increasing the alkalinity of purified water to prevent pipe corrosion and to restore essential nutrient levels.[122]
- In blast furnaces, limestone binds with silica and other impurities to remove them from the iron.[123]
- It can aid in the removal of toxic components created from coal burning plants and layers of polluted molten metals.[110]
Many limestone formations are porous and permeable, which makes them important petroleum reservoirs.[124] About 20% of North American hydrocarbon reserves are found in carbonate rock. Carbonate reservoirs are very common in the petroleum-rich Middle East,[59] and carbonate reservoirs hold about a third of all petroleum reserves worldwide.[125] Limestone formations are also common sources of metal ores, because their porosity and permeability, together with their chemical activity, promotes ore deposition in the limestone. The lead-zinc deposits of Missouri and the Northwest Territories are examples of ore deposits hosted in limestone.[59]
Scarcity
Limestone is a major industrial raw material that is in constant demand. This raw material has been essential in the iron and steel industry since the nineteenth century.[126] Companies have never had a shortage of limestone; however, it has become a concern as the demand continues to increase[127] and it remains in high demand today.[128] The major potential threats to supply in the nineteenth century were regional availability and accessibility.[126] The two main accessibility issues were transportation and property rights. Other problems were high capital costs on plants and facilities due to environmental regulations and the requirement of zoning and mining permits.[104] These two dominant factors led to the adaptation and selection of other materials that were created and formed to design alternatives for limestone that suited economic demands.[126]
Limestone was classified as a critical raw material, and with the potential risk of shortages, it drove industries to find new alternative materials and technological systems. This allowed limestone to no longer be classified as critical as replacement substances increased in production; minette ore is a common substitute, for example.[126]
Occupational safety and health
| NFPA 704 fire diamond | |
|---|---|
Limestone |
Powdered limestone as a food additive is generally recognized as safe[130] and limestone is not regarded as a hazardous material. However, limestone dust can be a mild respiratory and skin irritant, and dust that gets into the eyes can cause corneal abrasions. Because limestone contains small amounts of silica, inhalation of limestone dust could potentially lead to silicosis or cancer.[129]
United States
The Occupational Safety and Health Administration (OSHA) has set the legal limit (permissible exposure limit) for limestone exposure in the workplace as 15 mg/m3 (0.0066 gr/cu ft) total exposure and 5 mg/m3 (0.0022 gr/cu ft) respiratory exposure over an 8-hour workday. The National Institute for Occupational Safety and Health (NIOSH) has set a recommended exposure limit (REL) of 10 mg/m3 (0.0044 gr/cu ft) total exposure and 5 mg/m3 (0.0022 gr/cu ft) respiratory exposure over an 8-hour workday.[131]
Graffiti
Removing graffiti from weathered limestone is difficult because it is a porous and permeable material. The surface is fragile so usual abrasion methods run the risk of severe surface loss. Because it is an acid-sensitive stone some cleaning agents cannot be used due to adverse effects.[132]
Gallery
-
A stratigraphic section of Ordovician limestone exposed in central Tennessee, U.S. The less-resistant and thinner beds are composed of shale. The vertical lines are drill holes for explosives used during road construction.
-
Photo and etched section of a sample of fossiliferous limestone from the Kope Formation (Upper Ordovician) near Cincinnati, Ohio, U.S.
-
Fairborn, Ohio, U.S., showing grains mainly composed of crinoidfragments
-
Fossils in limestone from the northern Black Sea region
-
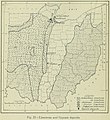
Limestone distribution in Ohio, from Geography of Ohio, 1923
-
Chalk is a variety of limestone. It is a softer, and more powdery material.
See also
- Coral sand
- Charmant Som
- In Praise of Limestone – Poem by W. H. Auden
- Kurkar – Regional name for an aeolian quartz calcrete on the Levantine coast
- Limepit – Old method of calcining limestone
- Sandstone – Type of sedimentary rock
- Agricultural lime – Soil additive containing calcium carbonate and other ingredients
- Liming (soil) – Application of minerals to soil
References
- ISBN 0-13-154728-3.
- ISBN 0-19-562816-0.
- ^ a b c d e f Boggs 2006, p. 159.
- ISBN 0-7167-2438-3.
- ISBN 0-922152-34-9.
- ISBN 0-13-642710-3.
- ^ Boggs 2006, p. 182-194.
- ^ Blatt, Middleton & Murray 1980, p. 448-449.
- ^ a b c d Blatt & Tracy 1996, p. 295.
- ^ Boggs 2006, p. 160.
- ^ Blatt, Middleton & Murray 1980, p. 467.
- ^ Blatt & Tracy 1996, pp. 301–302.
- ISBN 978-0-471-23896-6.
- ^ "Compressive strength test". Encyclopedia Britannica. Retrieved 4 February 2021.
- ^ Blatt & Tracy 1996, pp. 295–296.
- ^ Blatt, Middleton & Murray 1980, p. 452.
- ^ a b Blatt & Tracy 1996, pp. 295–300.
- ^ Blatt, Middleton & Murray 1980, p. 449.
- ^ Boggs 2006, p. 161-164.
- ^ a b Blatt & Tracy 1996, pp. 297–299.
- ^ Boggs 2006, pp. 164–165.
- .
- ^ a b c Blatt & Tracy 1996, p. 298.
- .
- S2CID 128851366.
- ^ Blatt & Tracy 1996, p. 299-300, 304.
- ^ Blatt, Middleton & Murray 1980, p. 460.
- ^ a b c Blatt & Tracy 1996, p. 300.
- ^ Boggs 2006, p. 166.
- ^ S2CID 134970335.
- ^ a b Boggs 2006, pp. 166–167.
- ^ Blatt & Tracy 1996, pp. 315–317.
- S2CID 129625906.
- ^ Blatt & Tracy 1996, pp. 474.
- ^ "Carbonate Classification: SEPM STRATA".
- ISBN 0-914696-14-9.
- ^ Dunham, R. J. (1962). "Classification of carbonate rocks according to depositional textures". In Ham, W. E. (ed.). Classification of Carbonate Rocks. American Association of Petroleum Geologists Memoirs. Vol. 1. pp. 108–121.
- .
- ^ Blatt, Middleton & Murray 1980, p. 479-480.
- ^ a b Boggs 2006, p. 172.
- ^ Boggs 2006, p. 177.
- ^ Boggs 2006, pp. 174–176.
- ISBN 0-08-086962-9.
- ^ Boggs 2006, pp. 176–182.
- .
- ^ Blatt, Middleton & Murray 1980, pp. 460–464.
- ^ Boggs 2006, p. 180.
- ^ Boggs 2006, pp. 177, 181.
- ^ Blatt, Middleton & Murray 1980, pp. 497–501.
- ^ ISSN 1788-2281.
- ^ Blatt, Middleton & Murray 1980, p. 497-503.
- ^ Blatt & Tracy 1996, p. 312.
- ^ Blatt, Middleton & Murray 1980, pp. 507–509.
- ^ a b Blatt & Tracy 1996, p. 312-316.
- ^ a b Boggs 2006, pp. 186–187.
- ^ S2CID 131159219.
- ^ a b Blatt, Middleton & Murray 1980, pp. 512–528.
- .
- ^ a b c d e Blatt, Middleton & Murray 1980, p. 445.
- ^ Blatt, Middleton & Murray 1980, p. 448.
- ^ Boggs 2006, p. 159-161.
- ^ Boggs 2006, p. 176-177.
- ^ Blatt, Middleton & Murray 1980, p. 446, 733.
- ^ Blatt, Middleton & Murray 1980, p. 468-470.
- ^ Blatt, Middleton & Murray 1980, p. 446-447.
- ^ Blatt & Tracy 1996, p. 306-307.
- ^ Blatt, Middleton & Murray 1980, p. 474-479.
- ^ Blatt & Tracy 1996, p. 308-309.
- S2CID 133211098.
- ^ Blatt, Middleton & Murray 1980, p. 480-482.
- ^ a b Blatt & Tracy 1996, p. 309-310.
- S2CID 131241083.
- ^ "Term 'evaporite'". Oilfield Glossary. Archived from the original on 31 January 2012. Retrieved 25 November 2011.
- ^ Boggs 2006, p. 662.
- ^ Blatt, Middleton & Murray 1980, pp. 446, 471–474.
- ^ Blatt, Middleton & Murray 1980, pp. 446–471.
- ^ Blatt & Tracy 1996, p. 304.
- ISBN 978-0-8493-7907-9. Archivedfrom the original on 10 May 2016.
- ^ Blatt & Tracy 1996, p. 307.
- ISBN 1-4443-0412-7. Retrieved 4 February 2021.
- ^ a b Blatt & Tracy 1996, pp. 307–308.
- .
- ISBN 0-19-857784-2. Retrieved 5 February 2021.
- ISBN 978-0-231-16057-5.
- ^ ISBN 0-471-86197-9.
- ^ "Karst Landscapes of Illinois: Dissolving Bedrock and Collapsing Soil". Prairie Research Institute. Illinois State Geological Survey. Archived from the original on 2 December 2020. Retrieved 26 December 2020.
- doi:10.1016/S0012-8252(02)00131-9. Archived from the original(PDF) on 25 March 2009.
- hdl:11441/137125. Retrieved 23 June 2016.
- ^ McNamara, M.; Hennessy, R. (2010). "The geology of the Burren region, Co. Clare, Ireland" (PDF). Project NEEDN, The Burren Connect Project. Ennistymon: Clare County Council. Retrieved 3 February 2021.
- ^ "Isle of Wight, Minerals" (PDF). Archived from the original (PDF) on 2 November 2006. Retrieved 8 October 2006.
- .
- .
- ^ Luczaj, John A. (2013). "Geology of the Niagara Escarpment in Wisconsin". Geoscience Wisconsin. 22 (1): 1–34. Retrieved 5 February 2021.
- JSTOR 1302317.
- ISSN 0866-708X.
- ISBN 978-90-481-3054-2.
- JSTOR 24319647.
- ^ Thorsten Jansson, Stora Alvaret, Lenanders Tryckeri, Kalmar, 1999
- ^ Laufeld, S. (1974). Silurian Chitinozoa from Gotland. Fossils and Strata. Universitetsforlaget.
- Bibcode:2014EGUGA..16...30P. Retrieved 5 February 2021.
- ^ ISBN 978-1-86239-294-6. Archivedfrom the original on 15 February 2017.
- ^ ISBN 978-3-527-61201-7.
- ^ "Welcome to the Limestone City". Archived from the original on 20 February 2008. Retrieved 13 February 2008.
- ^ ISBN 978-1-4113-4253-8.
- S2CID 129082854.
- ^ Schele, Linda; Miller, Mary Ellen. The Blood of Kings: Dynasty and Ritual in Maya Art. Kimbell Art Museum. p. 41.
- ISBN 1-139-17116-X
- ^ "Odessa catacombs". Odessa travel guide. Retrieved 13 June 2020.
- ISBN 0-7506-3898-2.
- ^ a b Bliss, J. D., Hayes, T. S., & Orris, G. J. (2012, August). Limestone—A Crucial and Versatile Industrial Mineral Commodity. Retrieved February 23, 2021, from https://pubs.usgs.gov/fs/2008/3089/fs2008-3089.pdf
- S2CID 94721996.
- ^ "Approaches in modeling the impact of air pollution-induced material degradation" (PDF). Archived from the original (PDF) on 16 July 2011. Retrieved 18 November 2010.
- .
- ^ Hatch, Jonathan (18 April 2018). "How to clean limestone". How to Clean Things. Saint Paul Media, Inc. Retrieved 5 February 2021.
- ISBN 978-3-527-61201-7.
- .
- ISBN 0-87335-233-5. Archivedfrom the original on 16 December 2017.
- ISBN 3-0348-9490-2.
- ^ Man, C.K.; Teacoach, K.A. (2009). "How does limestone rock dust prevent coal dust explosions in coal mines?" (PDF). Mining Engineering: 61. Retrieved 30 November 2020.
- ^ "Why Fortified Flour?". Wessex Mill. Retrieved 5 February 2021.
- ^ "A Guide to Giving Your Layer Hens Enough Calcium". Poultry One. Archived from the original on 3 April 2009.
- ^ "Nutrient minerals in drinking-water and the potential health consequences of consumption of demineralized and remineralized and altered mineral content drinking-water: Consensus of the meeting". World Health Organization report. Archived from the original on 24 December 2007.
- ISBN 0-901462-88-8.
- .
- ^ Boggs 2006, p. p=159.
- ^ S2CID 221052279.
- S2CID 221055042.
- ^ ResearchAndMarkets.com (9 June 2020). "Global Limestone Market Analysis and Forecasts 2020-2027 - Steady Growth Projected over the Next Few Years - ResearchAndMarkets.com". Limestone - Global Market Trajectory & Analytics. businesswire.com. Retrieved 24 March 2021.
- ^ a b Lhoist North America. "Material Safety Data Sheet: Limestone" (PDF). Retrieved 5 February 2021.
- ^ "CFR - Code of Federal Regulations Title 21". US Food & Drug Administration. US Department of Health & Human Services. Retrieved 5 February 2021.
- ^ "Limestone". NIOSH Pocket Guide to Chemical Hazards. CDC. Archived from the original on 20 November 2015. Retrieved 19 November 2015.
- ^ Weaver, Martin E. (October 1995). "Removing Graffiti from Historic Masonry". National Park Service. Retrieved 5 February 2019.
Further reading
- Boynton, Robert S. (1980). Chemistry and Technology of Lime and Limestone. Wiley. ISBN 0-471-02771-5.