Cold seep
| Marine habitats |
|---|
 |
| Coastal habitats |
| Ocean surface |
|
| Open ocean |
| Sea floor |
A cold seep (sometimes called a cold vent) is an area of the
Cold seeps develop unique topography over time, where reactions between methane and seawater create carbonate rock formations and reefs. These reactions may also be dependent on bacterial activity. Ikaite, a hydrous calcium carbonate, can be associated with oxidizing methane at cold seeps.
Types

Types of cold seeps can be distinguished according to the depth, as shallow cold seeps and deep cold seeps.[2] Cold seeps can also be distinguished in detail, as follows:
- oil/gas seeps[2]
- gas seeps:[2] methane seeps
- gas hydrate seeps[2]
- brine seeps[2] are formed in brine pools
- pockmarks[2]
- mud volcanoes[2]
Formation and ecological succession
Cold seeps occur over fissures on the seafloor caused by
Methane (CH
4) is the main component of
Chemosynthetic communities
Biological research in cold seeps and hydrothermal vents has been mostly focused on the
A community composition's orderly shift from one set of species to another is called ecological succession.[3]
The first type of organism to take advantage of this deep-sea energy source is

During this initial stage, when methane is relatively abundant, dense
This microbial activity produces
Cold seeps do not last indefinitely. As the rate of gas seepage slowly decreases, the shorter-lived, methane-hungry mussels (or more precisely, their methane-hungry bacterial symbionts) start to die off.[3] At this stage, tubeworms become the dominant organism in a seep community.[3] As long as there is some sulfide in the sediment, the sulfide-mining tubeworms can persist.[3] Individuals of one tubeworm species Lamellibrachia luymesi have been estimated to live for over 250 years in such conditions.[3]
 |
 |
 |
The Benthic Filter
The organisms living at cold seeps have a large impact on the carbon cycle and on climate. Chemosynthetic organisms, specifically methanogenic (methane-consuming) organisms, prohibit the methane seeping up from beneath the seafloor from being released into the water above. Since methane is such a potent greenhouse gas, methane release could cause global warming when gas hydrate reservoirs destabilized.[7] The consumption of methane by aerobic and anaerobic seafloor life is called “the benthic filter”.[8] The first part of this filter is the anaerobic bacteria and archaea underneath the seafloor that consume methane through the anaerobic oxidation of methane (AOM).[8] If the flux of methane flowing through the sediment is too large, and the anaerobic bacteria and archaea are consuming the maximum amount of methane, then the excess methane is consumed by free-floating or symbiotic aerobic bacteria above the sediment at the seafloor. The symbiotic bacteria have been found in organisms such as tube worms and clams living at cold seeps; these organisms provide oxygen to the aerobic bacteria as the bacteria provide energy they obtain from the consumption of methane. Understanding how efficient the benthic filter is can help predict how much methane escapes the seafloor at cold seeps and enters the water column and eventually the atmosphere. Studies have shown that 50-90% of methane is consumed at cold seeps with bacterial mats. Areas with clam beds have less than 15% of methane escaping.[7] Efficiency is determined by a number of factors. The benthic layer is more efficient with low flow of methane, and efficiency decreases as methane flow or the speed of flow increases.[8] Oxygen demand for cold seep ecosystems is much higher than other benthic ecosystems, so if the bottom water does not have enough oxygen, then the efficiency of aerobic microbes in removing methane is reduced.[7] The benthic filter cannot affect methane that is not traveling through the sediment. Methane can bypass the benthic filter if it bubbles to the surface or travels through cracks and fissures in the sediment.[7] These organisms are the only biological sink of methane in the ocean.[8]
Comparison with other communities

Cold seeps and
However, hydrothermal vents and cold seeps also differ in many ways. Compared to the more stable cold seeps, vents are characterized by locally-high temperatures, strongly fluctuating temperatures, pH, sulfide and oxygen concentrations, often the absence of sediments, a relatively young age, and often-unpredictable conditions, such as waxing and waning of vent fluids or volcanic eruptions.
End of cold seep community
Finally, as cold seeps become inactive, tubeworms also start to disappear, clearing the way for
Distribution
Cold seeps were discovered in 1983 by Charles Paull and colleagues on the Florida Escarpment in the
In addition to cold seeps existing today, the fossil remains of ancient seep systems have been found in several parts of the world. Some of these are located far inland in places formerly covered by
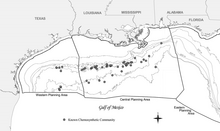
In the Gulf of Mexico

Discoveries
The chemosynthetic communities of the Gulf of Mexico have been studied extensively since the 1990s, and communities first discovered on the upper slope are likely the best understood seep communities in the world. The history of the discovery of these remarkable animals has all occurred since the 1980s. Each major discovery was unexpected―from the first hydrothermal vent communities anywhere in the world to the first cold seep communities in the Gulf of Mexico.[21]
Communities were discovered in the eastern Gulf of Mexico in 1983 using the crewed submersible
Distribution
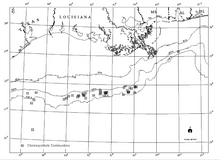
There is a clear relationship between known hydrocarbon discoveries at great depth in the Gulf slope and chemosynthetic communities, hydrocarbon seepage, and authigenic minerals including carbonates at the seafloor (Sassen et al., 1993a and b). While the hydrocarbon reservoirs are broad areas several kilometers beneath the Gulf, chemosynthetic communities occur in isolated areas with thin veneers of sediment only a few meters thick.[21]
The northern Gulf of Mexico slope includes a
The time scale for oil and gas migration from source systems is on the scale of millions of years (Sassen, 1997). Seepage from hydrocarbon sources through faults towards the surface tends to be diffused through the overlying sediment, carbonate outcroppings, and
In the upper slope environment, the hard substrates resulting from carbonate precipitation can have associated communities of non-chemosynthetic animals, including a variety of sessile
The widespread nature of Gulf of Mexico chemosynthetic communities was first documented during contracted investigations by the Geological and Environmental Research Group (GERG) of Texas A&M University for the Offshore Operators Committee (Brooks et al., 1986).
Chemosynthetic communities are not found on the
MacDonald et al. (1993 and 1996) have analyzed
The densest aggregations of chemosynthetic organisms have been found at water depths of around 500 m (1,640 ft) and deeper.
Stability
According to Sassen (1997) the role of
Through
Precipitation of
All chemosynthetic communities are located in water depths beyond the effect of severe storms, including hurricanes, and there would have been no alteration of these communities caused from surface storms, including
Biology

MacDonald et al. (1990) has described four general community types. These are communities dominated by
Individual lamellibrachid
Tubeworms are either male or female. One recent discovery indicates that the spawning of female Lamellibrachia appears to have produced a unique association with the large bivalve Acesta bullisi, which lives permanently attached to the anterior tube opening of the tubeworm, and feeds on the periodic egg release (Järnegren et al., 2005). This close association between the bivalves and tubeworms was discovered in 1984 (Boland, 1986) but not fully explained. Virtually all mature Acesta individuals are found on female rather than male tubeworms. This evidence and other experiments by Järnegren et al. (2005) seem to have solved this mystery.[21]
Growth rates for methanotrophic mussels at cold seep sites have been reported (Fisher, 1995).
Unlike mussel beds, chemosynthetic clam beds may persist as a visual surface phenomenon for an extended period without input of new living individuals because of low dissolution rates and low sedimentation rates. Most clam beds investigated by Powell (1995) were inactive. Living individuals were rarely encountered. Powell reported that over a 50-year timespan, local extinctions and recolonization should be gradual and exceedingly rare. Contrasting these inactive beds, the first community discovered in the Central Gulf of Mexico consisted of numerous actively-plowing clams. The images obtained of this community were used to develop length/frequency and live/dead ratios as well as spatial patterns (Rosman et al., 1987a).[21]
Extensive
Heterotrophic species at seep sites are a mixture of species unique to seeps (particularly
In the Atlantic Ocean
BT – Barbados trench
OR – Orenoque sectors
EP – El Pilar sector
NIG – Nigerian slope
GUI – Guiness area
REG – Regab pockmark.
Cold-seep communities in the western
The occurrence of chemosymbiotic
Cold seeps are also known from the Northern Atlantic Ocean,[2] even ranging into the Arctic Ocean, off Canada and Norway.[14]
Extensive faunal sampling has been conducted from 400 and 3,300 m (1,300–10,800 ft) in the Atlantic Equatorial Belt from the Gulf of Mexico to the Gulf of Guinea including the Barbados accretionary prism, the Blake Ridge diapir, and in the Eastern Atlantic from the Congo and Gabon margins and the recently-explored Nigeria margin during Census of Marine Life ChEss project. Of the 72 taxa identified at the species level, a total of 9 species or species complexes are identified as amphi-Atlantic.[12]
The Atlantic Equatorial Belt seep megafauna community structure is influenced primarily by depth rather than by geographic distance. The bivalves Bathymodiolinae (within Mytilidae) species or complexes of species are the most widespread in the Atlantic. The Bathymodiolus boomerang complex is found at the Florida escarpment site, the Blake Ridge diapir, the Barbados prism, and the Regab site of Congo. The Bathymodiolus childressi complex is also widely distributed along the Atlantic Equatorial Belt from the Gulf of Mexico across to the Nigerian Margin, although not on the Regab or Blake Ridge sites. The commensal polynoid Branchipolynoe seepensis is known from the Gulf of Mexico, Gulf of Guinea, and Barbados. Other species with distributions extending from the eastern to western Atlantic are: gastropod Cordesia provannoides, the shrimp Alvinocaris muricola, the galatheids Munidopsis geyeri and Munidopsis livida, and probably the holothurid Chiridota heheva.[12]
There have been found cold seeps also in the Amazon deepsea fan. High-resolution seismic profiles near the shelf edge show evidence of near-surface slumps and faulting 20–50 m (66–164 ft) in the subsurface and concentrations (about 500 m2 or 5,400 sq ft) of methane gas. Several studies (e.g., Amazon Shelf Study—AMASEDS, LEPLAC, REMAC, GLORIA, Ocean Drilling Program) indicate that there is evidence for gas seepage on the slope off the Amazon fan based on the incidence of bottom-simulating reflections (BSRs), mud volcanoes, pockmarks, gas in sediments, and deeper hydrocarbon occurrences. The existence of methane at relatively shallow depths and extensive areas of gas hydrates have been mapped in this region. Also, gas chimneys have been reported, and exploratory wells have discovered sub-commercial gas accumulations and pockmarks along fault planes. A sound geological and geophysical understanding of the Foz do Amazonas Basin is already available and used by the energy companies.[28]
Exploration of new areas, such as potential seep sites off of the east coast of the U.S. and the
In the Mediterranean
The first biological evidence for reduced environments in the
During these first exploratory dives, symbiont-bearing taxa that are similar to those observed on the Olimpi and Anaximander mud fields were sampled and identified. This similarity is not surprising, as most of these taxa were originally described from dredging in the Nile fan.
In the Indian Ocean
In the Makran Trench, a subduction zone along the northeastern margin of the Gulf of Oman adjacent to the southwestern coast of Pakistan and the southeastern coast of Iran, compression of an accretionary wedge has resulted in the formation of cold seeps and mud volcanoes.[32]
In the West Pacific
Native
Japan
| Cold seep | |
| Hydrothermal vent | |
| Whale fall |
|
Deep sea communities around Japan are mainly researched by
Methane seep communities in Japan are distributed along plate convergence areas because of the accompanying tectonic activity. Many seeps have been found in the Japan Trench, Nankai Trough, Ryukyu Trench, Sagami Bay, Suruga Bay, and the Sea of Japan.[35]
Members of cold seep communities are similar to other regions in terms of family or genus, such as Polycheata, Lamellibrachia, Bivalavia, Solemyidae, Bathymodiolus in Mytilidae, Thyasiridae, Calyptogena in Vesicomyidae, and so forth.[34] Many of species in cold seeps of Japan are endemic.[35]
In Kagoshima Bay, there are methane gas seepages called "tagiri" (boiling). Lamellibrachia satsuma live around there. The depth of this site is only 80 m, which is the shallowest point where Siboglinidae are known to live. L. satsuma may be kept in an aquarium for a long period at 1 atm. Two aquariums in Japan are keeping and displaying L. satsuma. An observation method to introduce it into a transparent vinyl tube is being developed.[36]

DSV Shinkai 6500 discovered vesicomyid clam communities in the Southern Mariana Forearc. They depend on methane, which originates in serpentinite. Other chemosynthetic communities would depend on hydrocarbon origins organic substance in crust, but these communities depend on methane originating from inorganic substances from the mantle.[37][38]
In 2011, the area around the Japan Trench suffered from Tōhoku earthquake. There are cracks, methane seepages, and bacterial mats which were probably created by the earthquake.[39][40]
New Zealand
Off the mainland coast of
In the East Pacific

In the deep sea, the
Cold seeps (
Cold seeps with chemosynthetic communities along the USA Pacific coast occur in
Additionally, seeps have been discovered offshore southern California in the inner California Borderlands along several fault systems including the San Clemente fault,[53] San Pedro fault,[54] and San Diego Trough fault.[55] Fluid flow at the seeps along the San Pedro and San Diego Trough faults appears controlled by localized restraining bends in the faults.[55]
In the Antarctic
The first cold seep from the Southern Ocean was reported in 2005.[16] The relatively few investigations to the Antarctic deep sea have shown the presence of deep-water habitats, including hydrothermal vents, cold seeps, and mud volcanoes.[56] Other than the Antarctic Benthic Deep-Sea Biodiversity Project (ANDEEP) cruises, little work has been done in the deep sea.[56] There are more species waiting to be described.[56]
Detection
With continuing experience, particularly on the upper continental slope in the Gulf of Mexico, the successful prediction of the presence of tubeworm communities continues to improve; however, chemosynthetic communities cannot be reliably detected directly using
Fossilized records

Cold seep deposits are found throughout the
Environmental impacts
Major threats that cold seep ecosystems and their communities face today are seafloor litter, chemical contaminants, and climate change. Seafloor litter alters the habitat by providing hard substrate where none was available before or by overlying the sediment, thereby inhibiting gas exchange and interfering with organisms on the bottom of the sea. Studies of marine litter in the Mediterranean include surveys of seabed debris on the continental shelf, slope, and bathyal plain.[62][63] In most studies, plastic items accounted for much of the debris, sometimes as much as 90% or more of the total, owing to their ubiquitous use and poor degradability.
Weapons and bombs have also been discarded at sea, and their dumping in open waters contributes to seafloor contamination. Another major threat to the benthic fauna is the presence of lost fishing gear, such as nets and longlines, which contribute to ghost fishing and can damage fragile ecosystems such as cold-water corals.
Chemical contaminants such as
Climate-driven processes and climate change will affect the frequency and intensity of cascading, with unknown effects on the benthic fauna. Another potential effect of climate change is related to energy transport from surface waters to the seafloor.[67] Primary production will change in the surface layers according to sun exposure, water temperature, major stratification of water masses, and other effects, and this will affect the food chain down to the deep seafloor, which will be subject to differences in quantity, quality, and timing of organic matter input. As commercial fisheries move into deeper waters, all of these effects will affect the communities and populations of organisms in cold seeps and the deep sea in general.
See also
References
This article incorporates a
- ISBN 978-4-486-01787-5. p. 20.
- ^ PMID 20805986.
- ^ NOAAOcean Explorer | Lophelia II 2010: Oil Seeps and Deep Reefs | 18 October Log. Retrieved 25 January 2011.
- ^ PMID 22496753.
- ^ PMID 21976991.
- ^ PMID 15760275.
- ^ S2CID 54695808.
- ^ ISSN 1752-0894.
- .
- ISBN 978-1-4020-8461-4. Pages 204-205.
- S2CID 45699993.
- ^ PMID 20700528.
- ^ "Demise of Antarctic Ice Shelf Reveals New Life". National Science Foundation. 2007. Retrieved 14 February 2008.
- ^ ISBN 9780849335976.
- ^ "Pythias Oasis: An Underwater Spring Unlike Any Other". OOI Regional Cable Array. University of Washington. 1 October 2019. Retrieved 24 April 2023.
- ^ S2CID 35944740.
- hdl:10533/129437.
- ^ "Red List – Submarine structures made by leaking gases" (PDF). HELCOM. 2013. Retrieved 16 June 2017.
- .
- .
- ^ a b c d e f g h i j k l m n o p q r s t u v w x y z aa ab ac ad ae af ag ah ai aj ak al am an ao ap aq ar "Gulf of Mexico OCS Oil and Gas Lease Sales: 2007–2012. Western Planning Area Sales 204, 207, 210, 215, and 218. Central Planning Area Sales 205, 206, 208, 213, 216, and 222. Draft Environmental Impact Statement. Volume I: Chapters 1–8 and Appendices" (PDF). Minerals Management Service Gulf of Mexico OCS Region, New Orleans. U.S. Department of the Interior. November 2006. pp. 3–27, 3–31. Archived from the original (PDF) on 26 March 2009.
- ISBN 9781118668412. Archived from the originalon 28 October 2012. Retrieved 26 March 2012.
- S2CID 140681313.
- .
- )
- ^ I. R. McDonald, ed. (1998). "Stability and Change in Gulf of Mexico Chemosynthetic Communities" (PDF). U.S. Department of the Interior: OCS Study MMS 98-0034: Prepared by the Geochemical and Environmental Research Group: Texas A&M University. Archived from the original (PDF) on 29 December 2016. Retrieved 17 July 2016.
- .
- ^ PMID 21304960.
- ^ PMID 20689848.
- ^ Southward E., Andersen A., Hourdez S. (submitted 2010). "Lamellibrachia anaximandri n.sp., a new vestimentiferan tubeworm from the Mediterranean (Annelida)". Zoosystema.
- .
- ^ Fischer, D. ; Bohrmann, G. ; Zabel, M. ; Kasten, S. (April 2009): Geochemical zonation and characteristics of cold seeps along the Makran continental margin off Pakistan EGU General Assembly Conference Abstracts. Retrieved 19 November 2020.
- ^ .
- ^ ISBN 978-4-486-01787-5.
- ^ PMID 20689840.
- ^ Miyake, Hiroshi; Jun HASHIMOTO; Shinji TSUCHIDA (2010). "Observation method of behaviour of vestimentifean tube-worm (Lamellibrachia satsuma) in its tube" (PDF). JAMSTEC深海研究. (16-I.生物学編). Retrieved 30 March 2012.
- ^ "マリアナ海溝、チャレンジャー海淵の近くにおいて、マントル物質から栄養を摂る生態系を発見~有人潜水調査船「しんかい6500」による成果~". 7 February 2012. Archived from the original on 23 September 2012. Retrieved 29 March 2012.
- PMID 22323611.
- ^ "東北地方太平洋沖地震震源海域での有人潜水調査船「しんかい6500」による潜航調査で得られた画像について(速報)". 海洋研究開発機構. 15 August 2011. Retrieved 29 March 2012.
- PMID 22355782.
- ^ PMID 20689846.
- .
- .
- ^ .
- hdl:10261/56612.
- ^ .
- S2CID 85948533.
- ^ S2CID 121829940.
- ^ .
- .
- ^ Goffredi S. K. & Barry J. P. (2000). "Factors regulating productivity in chemoautotrophic symbioses; with emphasis on Calyptogena kilmeri and Calyptogena pacifica". Poster, Monterey Bay Aquarium Research Institute. accessed 3 February 2011. PDF.
- .
- S2CID 4310057.
- .
- ^ .
- ^ PMID 20689841.
- S2CID 230530430. Retrieved 15 March 2023.
- .
- . Retrieved 16 November 2022.
- hdl:11380/1119044.
- .
- .
- .
- .
- .
- PMID 18501382.
- PMID 19901326.
Further reading
- Bright, M.; Plum, C.; Riavitz, L. A.; Nikolov, N.; Martínez Arbizu, P.; Cordes, E. E.; Gollner, S. (2010). "Epizooic metazoan meiobenthos associated with tubeworm and mussel aggregations from cold seeps of the Northern Gulf of Mexico". Deep-Sea Research Part II: Topical Studies in Oceanography. 57 (21–23): 1982–1989. PMID 21264038.
- German, C. R.; Ramirez-Llodra, E.; Baker, M. C.; Tyler, P. A.; the PMID 21829722.
- Lloyd, K. G.; Albert, D. B.; Biddle, J. F.; Chanton, J. P.; Pizarro, O.; Teske, A. (2010). "Spatial Structure and Activity of Sedimentary Microbial Communities Underlying a Beggiatoa spp. Mat in a Gulf of Mexico Hydrocarbon Seep". PMID 20090951.
- Metaxas, A.; Kelly, N. E. (2010). "Do Larval Supply and Recruitment Vary among Chemosynthetic Environments of the Deep Sea?". PMID 20657831.
- Rodríguez, E.; Daly, M. (2010). "Phylogenetic Relationships among Deep-Sea and Chemosynthetic Sea Anemones: Actinoscyphiidae and Actinostolidae (Actiniaria: Mesomyaria)". PMID 20532040.
- Sibuet, M.; Olu, K. (1998). "Biogeography, biodiversity and fluid dependence of deep-sea cold-seep communities at active and passive margins". Deep-Sea Research Part II: Topical Studies in Oceanography. 45 (1–3): 517–567. .
- Vinn, O.; Hryniewicz, K; Little, C.T.S.; Nakrem, H.A. (2014). "A Boreal serpulid fauna from Volgian-Ryazanian (latest Jurassic-earliest Cretaceous) shelf sediments and hydrocarbon seeps from Svalbard". Geodiversitas. 36 (4): 527–540. S2CID 129587761. Retrieved 9 January 2014.
- Vinn, O.; Kupriyanova, E.K.; Kiel, S. (2013). "Serpulids (Annelida, Polychaeta) at Cretaceous to modern hydrocarbon seeps: Ecological and evolutionary patterns". Palaeogeography, Palaeoclimatology, Palaeoecology. 390: 35–41. . Retrieved 9 January 2014.


