Marine primary production
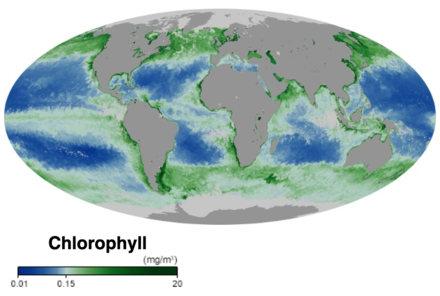
Marine primary production is the chemical synthesis in the ocean of
Most marine primary production is generated by a diverse collection of
Primary production in the ocean can be contrasted with primary production on land. Globally the ocean and the land each produce about the same amount of primary production, but in the ocean primary production comes mainly from cyanobacteria and algae, while on land it comes mainly from vascular plants.
Marine algae includes the largely invisible and often
| Part of a series of overviews on |
| Marine life |
|---|
 |
Marine primary producers
click to animate
• Red = diatoms (big phytoplankton, which need silica)
• Yellow = flagellates (other big phytoplankton)
• Green = prochlorococcus (small phytoplankton that cannot use nitrate)
• Cyan = synechococcus (other small phytoplankton)
Opacity indicates concentration of the carbon biomass. In particular, the role of the swirls and filaments (mesoscale features) appear important in maintaining high biodiversity in the ocean.[2][3]
| Part of a series on the |
| Carbon cycle |
|---|
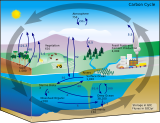 |
The principal marine primary producers are cyanobacteria, algae and marine plants. The oxygen released as a by-product of photosynthesis is needed by nearly all living things to carry out cellular respiration. In addition, primary producers are influential in the global carbon and water cycles. They stabilize coastal areas and can provide habitats for marine animals. The term division has been traditionally used instead of phylum when discussing primary producers, although the International Code of Nomenclature for algae, fungi, and plants now accepts the terms as equivalent.[5]
In a reversal of the pattern on land, in the oceans, almost all photosynthesis is performed by algae and
Unlike terrestrial ecosystems, the majority of primary production in the ocean is performed by free-living
The factors limiting primary production in the ocean are also very different from those on land. The availability of water, obviously, is not an issue (though its
In 2020 researchers reported that measurements over the last two decades of primary production in the
Cyanobacteria

The first primary producers that used photosynthesis were oceanic
The tiny marine cyanobacterium Prochlorococcus, discovered in 1986, forms today part of the base of the ocean food chain and accounts for more than half the photosynthesis of the open ocean[23] and an estimated 20% of the oxygen in the Earth's atmosphere.[24] It is possibly the most plentiful genus on Earth: a single millilitre of surface seawater may contain 100,000 cells or more.[25]
Originally, biologists thought

Biological pigments
Chloroplasts

Chloroplasts (from the Greek chloros for green, and plastes for "the one who forms"[31]) are organelles that conduct photosynthesis, where the photosynthetic pigment chlorophyll captures the energy from sunlight, converts it, and stores it in the energy-storage molecules while freeing oxygen from water in plant and algal cells. They then use the stored energy to make organic molecules from carbon dioxide in a process known as the Calvin cycle.
A chloroplast is a type of organelle known as a
Most chloroplasts can probably be traced back to a single
Microbial rhodopsin

(2) it changes its configuration so a proton is expelled from the cell
(3) the chemical potential causes the proton to flow back to the cell
(4) thus generating energy
(5) in the form of adenosine triphosphate.[34]
Phototrophic metabolism relies on one of three energy-converting pigments: chlorophyll, bacteriochlorophyll, and retinal. Retinal is the chromophore found in rhodopsins. The significance of chlorophyll in converting light energy has been written about for decades, but phototrophy based on retinal pigments is just beginning to be studied.[35]
| External videos | |
|---|---|
In 2000 a team of microbiologists led by Edward DeLong made a crucial discovery in the understanding of the marine carbon and energy cycles. They discovered a gene in several species of bacteria[37][38] responsible for production of the protein rhodopsin, previously unheard of in bacteria. These proteins found in the cell membranes are capable of converting light energy to biochemical energy due to a change in configuration of the rhodopsin molecule as sunlight strikes it, causing the pumping of a proton from inside out and a subsequent inflow that generates the energy.[39] The archaeal-like rhodopsins have subsequently been found among different taxa, protists as well as in bacteria and archaea, though they are rare in complex multicellular organisms.[40][41][42]
Research in 2019 shows these "sun-snatching bacteria" are more widespread than previously thought and could change how oceans are affected by global warming. "The findings break from the traditional interpretation of marine ecology found in textbooks, which states that nearly all sunlight in the ocean is captured by chlorophyll in algae. Instead, rhodopsin-equipped bacteria function like hybrid cars, powered by organic matter when available — as most bacteria are — and by sunlight when nutrients are scarce."[43][35]
There is an astrobiological conjecture called the Purple Earth hypothesis which surmises that original life forms on Earth were retinal-based rather than chlorophyll-based, which would have made the Earth appear purple instead of green.[44][45]
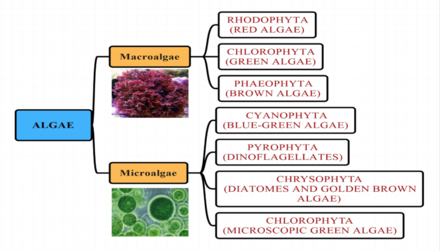
Marine algae
| Part of a series on |
| Plankton |
|---|
 |
Algal groups
Marine algae have traditionally been placed in groups such as: green algae, red algae, brown algae, diatoms, coccolithophores and dinoflagellates.
Green algae
Green algae live most of their lives as single cells or are filamentous, while others form colonies made up from long chains of cells, or are highly differentiated macroscopic seaweeds. They form an informal group containing about 8,000 recognized species.[47]
Red algae
Modern
-
Cyanidiophyceae colony, a class of unicellular red algae
-
The seaweed Porphyra umbilicalis
Brown algae
Diatoms
Altogether, about 45 percent of the
-
Diatoms are one of the most common types of phytoplankton
-
They are a major algae group generating about 20% of world oxygen production.[52]
-
Diatoms have glass like cell walls calledsilica.[53]
Coccolithophores
-
The ubiquitous Emiliania huxleyi
-
Emiliania huxleyi bloom off south England
Dinoflagellate
-
Dinoflagellates
-
Karenia brevis produces red tides highly toxic to humans[58]
-
Red tide
Mixotrophic algae
Other groups
-
Diplonemidsmay be abundant in the world oceans
Traditionally the
Between 2009 and 2013, the
By size
Algae can be classified by size as microalgae or macroalgae.
Microalgae
- Microalgae
-
Zooxanthellae is a photosynthetic algae that lives inside hosts like coral
-
A single-celledendosymbiotically
-
Euglena mutabilis, a photosynthetic flagellate
Macroalgae

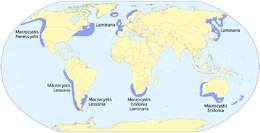
- Macroalgae
-
Giant kelpis technically a protist since it is not a true plant, yet it is multicellular and can grow to 50 m
-
Sargassum seaweed is a brown alga with air bladders that help it float
-
Sargassum fish are camouflaged to live among drifting Sargassum seaweed
-
This unicellulartidal zones. It can have a 4 cm diameter.[68]
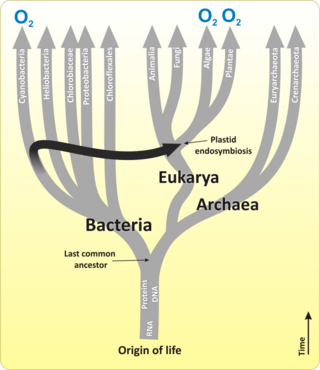
Evolution of land plants
The diagram on the right shows an evolutionary scenario for the conquest of land by streptophytes.
The earliest land plants probably interacted with beneficial
Marine plants
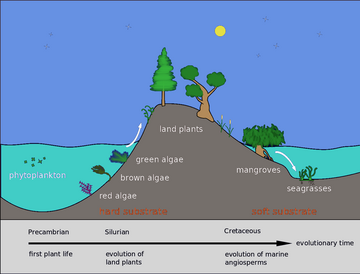
Back in the
Plant life can flourish in the brackish waters of
Light is only able to penetrate the top 200 metres (660 ft) so this is the only part of the sea where plants can grow.[77] The surface layers are often deficient in biologically active nitrogen compounds. The marine nitrogen cycle consists of complex microbial transformations which include the fixation of nitrogen, its assimilation, nitrification, anammox and denitrification.[78] Some of these processes take place in deep water so that where there is an upwelling of cold waters, and also near estuaries where land-sourced nutrients are present, plant growth is higher. This means that the most productive areas, rich in plankton and therefore also in fish, are mainly coastal.[79]: 160–163
Mangroves
Mangroves provide important nursery habitats for marine life, acting as hiding and foraging places for larval and juvenile forms of larger fish and invertebrates. Based on satellite data, the total world area of mangrove forests was estimated in 2010 as 134,257 square kilometres (51,837 sq mi).[80][81]
- Spalding, M. (2010) World atlas of mangroves, Routledge. .
Seagrasses
Like mangroves, seagrasses provide important nursery habitats for larval and juvenile forms of larger fish and invertebrates. The total world area of seagrass meadows is more difficult to determine than mangrove forests, but was conservatively estimated in 2003 as 177,000 square kilometres (68,000 sq mi).[82]
-
Sea dragons camouflaged to look like floating seaweed live in kelp forests and seagrass meadows[83]
| External videos | |
|---|---|
Stoichiometry
The
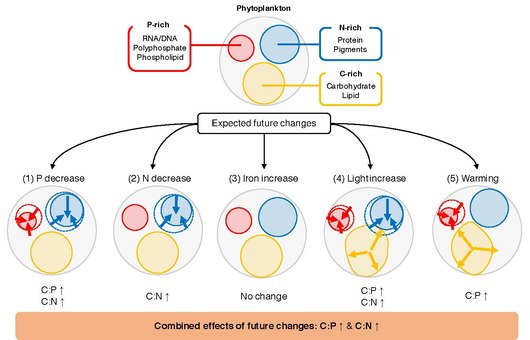
to major environmental drivers
A key unresolved question is what determines C:N:P of individual phytoplankton. Phytoplankton grows in the
Meta-analysis/systematic review is a powerful statistical framework for synthesizing and integrating research results obtained from independent studies and for uncovering general trends.[100] The seminal synthesis by Geider and La Roche in 2002,[101] as well as the more recent work by Persson et al. in 2010,[102] has shown that C:P and N:P could vary by up to a factor of 20 between nutrient-replete and nutrient-limited cells. These studies have also shown that the C:N ratio can be modestly plastic due to nutrient limitation. A meta-analysis study by Hillebrand et al. in 2013 highlighted the importance of growth rate in determining elemental stoichiometry and showed that both C:P and N:P ratios decrease with the increasing growth rate.[103] In 2015, Yvon-Durocher et al. investigated the role of temperature in modulating C:N:P.[104] Although their dataset was limited to studies conducted prior to 1996, they have shown a statistically significant relationship between C:P and temperature increase. MacIntyre et al. (2002)[105] and Thrane et al. (2016)[106] have shown that irradiance plays an important role in controlling optimal cellular C:N and N:P ratios. Most recently, Moreno and Martiny (2018) provided a comprehensive summary of how environmental conditions regulate cellular stoichiometry from a physiological perspective.[93][84]
The elemental stoichiometry of marine phytoplankton plays a critical role in global biogeochemical cycles through its impact on nutrient cycling, secondary production, and carbon export. Although extensive laboratory experiments have been carried out over the years to assess the influence of different environmental drivers on the elemental composition of phytoplankton, a comprehensive quantitative assessment of the processes is still lacking. Here, the responses of P:C and N:C ratios of marine phytoplankton have been synthesized to five major drivers (inorganic phosphorus, inorganic nitrogen, inorganic iron, irradiance, and temperature) by a meta-analysis of experimental data across 366 experiments from 104 journal articles. These results show that the response of these ratios to changes in macronutrients is consistent across all the studies, where the increase in nutrient availability is positively related to changes in P:C and N:C ratios. The results show that eukaryotic phytoplankton are more sensitive to the changes in macronutrients compared to prokaryotes, possibly due to their larger cell size and their abilities to regulate their gene expression patterns quickly. The effect of irradiance was significant and constant across all studies, where an increase in irradiance decreased both P:C and N:C. The P:C ratio decreased significantly with warming, but the response to temperature changes was mixed depending on the culture growth mode and the growth phase at the time of harvest. Along with other oceanographic conditions of the subtropical gyres (e.g., low macronutrient availability), the elevated temperature may explain why P:C is consistently low in subtropical oceans. Iron addition did not systematically change either P:C or N:C.[84]
Evolutionary timeline


See also
- Algae
- Aquatic plants
- Biological pump
- Evolutionary history of plants
- Oceanic carbon cycle
- Plant evolution
- Timeline of plant evolution
- Evolution of photosynthesis
References
- ^ Chlorophyll NASA Earth Observatory. Accessed 30 November 2019.
- ^ Modeled Phytoplankton Communities in the Global Ocean NASA Hyperwall, 30 September 2015.
 This article incorporates text from this source, which is in the public domain.
This article incorporates text from this source, which is in the public domain.
- ^ Darwin Project Massachusetts Institute of Technology.
- ^ ISBN 978-0-321-54325-7.
- ^ McNeill, J.; et al., eds. (2012). International Code of Nomenclature for algae, fungi, and plants (Melbourne Code), Adopted by the Eighteenth International Botanical Congress Melbourne, Australia, July 2011 (electronic ed.). International Association for Plant Taxonomy. Retrieved 14 May 2017.
- PMID 10477309.
- ^ Roach, John (7 June 2004). "Source of Half Earth's Oxygen Gets Little Credit". National Geographic News. Archived from the original on 8 June 2004. Retrieved 4 April 2016.
- S2CID 10267488.
- ^ Sigman, D.M.; Hain, M.P. (2012). "The Biological Productivity of the Ocean" (PDF). Nature Education Knowledge. 3 (6): 1–16. Retrieved 1 June 2015.
The deep chlorophyll maximum (DCM) occurs at the contact where there is adequate light for photosynthesis and yet significant nutrient supply from below.
- PMID 24143135.
- ^ "A 'regime shift' is happening in the Arctic Ocean, scientists say". phys.org. Retrieved 16 August 2020.
- S2CID 220433818. Retrieved 16 August 2020.
- .
- .
- .
- .
- .
- .
- ISBN 978-0-08-087782-2.
- ^ "The Rise of Oxygen". Astrobiology Magazine. 30 July 2003. Archived from the original on 3 April 2015. Retrieved 6 April 2016.
{{cite web}}: CS1 maint: unfit URL (link) - S2CID 53618061.
- ^ Rothschild, Lynn (September 2003). "Understand the evolutionary mechanisms and environmental limits of life". NASA. Archived from the original on 29 March 2012. Retrieved 13 July 2009.
- PMID 14631732. Archived from the original(PDF) on 19 April 2014. Retrieved 11 July 2019.
- ^ "The Most Important Microbe You've Never Heard Of". npr.org.
- PMID 23703908.
- ISBN 978-0-8053-4416-5.
- ^ Allaby, M., ed. (1992). "Algae". The Concise Dictionary of Botany. Oxford: Oxford University Press.
- PMID 16669781.
- ^ Lee, DW (2007) Nature's palette - the science of plant color. University of Chicago Press
- ^ Concepts of Biology: Eukaryotic Origins. OpenStax CNX. Retrieved 16 July 2020.
 Modified text was copied from this source, which is available under a Creative Commons Attribution 4.0 International License.
Modified text was copied from this source, which is available under a Creative Commons Attribution 4.0 International License.
- ^ "chloroplast". Online Etymology Dictionary.
- ^ Basic Biology (18 March 2016). "Bacteria".
- PMID 21652304.
- PMID 20436957. e1000359.
- ^ PMID 31457093.
- PMID 19709178.
- S2CID 1461255.
- American Academy of Microbiology. Archived from the originalon 7 August 2016. Retrieved 2 July 2016.
- ^ Bacteria with Batteries, Popular Science, January 2001, Page 55.
- PMID 10988064.
- PMID 26286928.
- ISBN 978-4-431-55516-2. Retrieved 30 September 2015.
- ^ A tiny marine microbe could play a big role in climate change University of Southern California, Press Room, 8 August 2019.
- S2CID 119341330.
- Bibcode:2006AAS...209.0605S.
- ISBN 978-93-88170-77-2..
 Modified text was copied from this source, which is available under a Creative Commons Attribution 4.0 International License
Modified text was copied from this source, which is available under a Creative Commons Attribution 4.0 International License - S2CID 30911529.
- ^ a b Guiry, M.D.; Guiry, G.M. (2016). "Algaebase". www.algaebase.org. Retrieved 20 November 2016.
- ISBN 978-0-565-09175-0.
- OCLC 443576944.
- S2CID 16849373.
- ^ The Air You're Breathing? A Diatom Made That
- ^ "More on Diatoms". University of California Museum of Paleontology. Archived from the original on 4 October 2012. Retrieved 11 July 2019.
- ^ This twilight zone is dark, watery, and yes, also full of intrigue NASA Blog, 21 August 2018.
- PMID 22615387
- ^ "Biogeography and dispersal of micro-organisms: a review emphasizing protists", Acta Protozoologica, 45 (2): 111–136, 2005
- PMID 36733478.
- PMID 8282683.
- .
- PMID 27875688.
- doi:10.12688/f1000research.8040.1..
 Modified text was copied from this source, which is available under a Creative Commons Attribution 4.0 International License
Modified text was copied from this source, which is available under a Creative Commons Attribution 4.0 International License - .
- ^ Starckx, Senne (31 October 2012) A place in the sun - Algae is the crop of the future, according to researchers in Geel Archived 4 March 2016 at the Wayback Machine Flanders Today, Retrieved 8 December 2012
- .
- ^ Mann, K.H. 1973. Seaweeds: their productivity and strategy for growth. Science 182: 975-981.
- ISBN 978-1-4053-3308-5.
- ISBN 978-1-58544-617-9.
- ^ PMID 31922568..
 Modified text was copied from this source, which is available under a Creative Commons Attribution 4.0 International License
Modified text was copied from this source, which is available under a Creative Commons Attribution 4.0 International License - PMID 29463716.
- S2CID 207844920.
- 2.0.CO;2]
- PMID 21283684.
- ^ "Mangal (Mangrove)". Mildred E. Mathias Botanical Garden. Retrieved 11 July 2013.
- ^ "Coastal Salt Marsh". Mildred E. Mathias Botanical Garden. Retrieved 11 July 2013.
- ^ "Facts and figures on marine biodiversity". Marine biodiversity. UNESCO. 2012. Retrieved 11 July 2013.
- ^ Russell, F. S.; Yonge, C. M. (1928). The Seas. Frederick Warne. pp. 225–227.
- PMID 23713119.
- ISBN 978-0-19-860687-1.
- ISBN 9780520240476
- ^ Froese, Rainer; Pauly, Daniel (eds.) (2009). "Phycodurus eques" in FishBase. July 2009 version.
- ^ S2CID 226197209..
 Modified text was copied from this source, which is available under a Creative Commons Attribution 4.0 International License
Modified text was copied from this source, which is available under a Creative Commons Attribution 4.0 International License - .
- S2CID 95940597.
- ^ Redfield A. C. (1958) "The biological control of chemical factors in the environment", American Scientist, 46(3) 230A–221.
- S2CID 4325136.
- .
- PMID 30451846.
- S2CID 5677709.
- S2CID 13747201.
- ^ PMID 28853998.
- S2CID 15511444.
- .
- .
- PMID 25902497.
- S2CID 201730744.
- PMID 32025278.
- S2CID 3761687.
- S2CID 13747201.
- .
- S2CID 55496787.
- .
- S2CID 29301640.
- PMID 27250733.
- doi:10.1186/s40168-018-0445-0..
 Modified text was copied from this source, which is available under a Creative Commons Attribution 4.0 International License
Modified text was copied from this source, which is available under a Creative Commons Attribution 4.0 International License - doi:10.3852/09-016.
- .
Further reading
- ISBN 9781468438901.
- Falkowski, Paul and ISBN 9781400849727.
- Falkowski P and Knoll AH (2011) Evolution of Primary Producers in the Sea Academic Press. ISBN 9780080550510.
- Kirk, John T. O. (2010) Light and Photosynthesis in Aquatic Ecosystems Third edition revised, Cambridge University Press. ISBN 9781139493918.



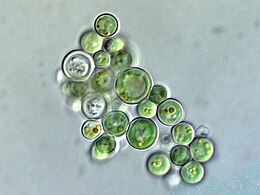


![They are a major algae group generating about 20% of world oxygen production.[52]](http://upload.wikimedia.org/wikipedia/commons/thumb/3/31/Diatoms_through_the_microscope.jpg/297px-Diatoms_through_the_microscope.jpg)
![Diatoms have glass like cell walls called frustules which are made of silica.[53]](http://upload.wikimedia.org/wikipedia/commons/thumb/9/90/Diatom_algae_Amphora_sp.jpg/260px-Diatom_algae_Amphora_sp.jpg)
![Diatoms linked in a colonial chain [54]](http://upload.wikimedia.org/wikipedia/commons/thumb/b/b2/Phytoplankton_in_the_form_of_a_diatom_chain.jpg/260px-Phytoplankton_in_the_form_of_a_diatom_chain.jpg)



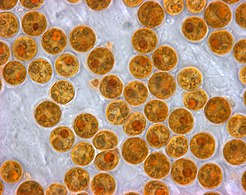
![Karenia brevis produces red tides highly toxic to humans[58]](http://upload.wikimedia.org/wikipedia/commons/thumb/a/a0/Karenia_brevis.jpg/223px-Karenia_brevis.jpg)






![This unicellular bubble algae lives in tidal zones. It can have a 4 cm diameter.[68]](http://upload.wikimedia.org/wikipedia/commons/thumb/3/3d/Ventricaria_ventricosa.JPG/248px-Ventricaria_ventricosa.JPG)


![Sea dragons camouflaged to look like floating seaweed live in kelp forests and seagrass meadows[83]](http://upload.wikimedia.org/wikipedia/commons/thumb/2/2a/Leafy_Sea_Dragon_SA.jpg/301px-Leafy_Sea_Dragon_SA.jpg)

