Skeletal muscle
| Skeletal muscle | |
|---|---|
 Depiction of skeletal muscle | |
| Details | |
| Synonyms | Skeletal striated muscle, striated voluntary muscle |
| System | Muscular system |
| Identifiers | |
| Latin | muscularis skeletalis |
| MeSH | D018482 |
| TA2 | 1976 |
| TH | H2.00.05.2.00002 |
| Anatomical terminology | |
Skeletal muscle (commonly referred to as muscle) is one of the three types of
A skeletal muscle contains multiple
Muscle fibers are in turn composed of
Skeletal muscle comprises about 35% of the body of humans by weight.[7] The functions of skeletal muscle include producing movement, maintaining body posture, controlling body temperature, and stabilizing joints.[8] Skeletal muscle is also an endocrine organ.[9][10][11] Under different physiological conditions, subsets of 654 different proteins as well as lipids, amino acids, metabolites and small RNAs are found in the secretome of skeletal muscles.[12]
Skeletal muscles are substantially composed of
Considerable research on skeletal muscle is focused on the muscle fiber cells, the myocytes, as discussed in detail in the first sections, below. Recently, interest has also focused on the different types of mononuclear cells of skeletal muscle, as well as on the endocrine functions of muscle, described subsequently, below.
Structure
Gross anatomy
There are more than 600 skeletal muscles in the human body, making up around 40% of body weight in healthy young adults.[18][19][20] In Western populations, men have on average around 61% more skeletal muscle than women.[21] Most muscles occur in bilaterally-placed pairs to serve both sides of the body. Muscles are often classed as groups of muscles that work together to carry out an action. In the torso there are several major muscle groups including the pectoral, and abdominal muscles; intrinsic and extrinsic muscles are subdivisions of muscle groups in the hand, foot, tongue, and extraocular muscles of the eye. Muscles are also grouped into compartments including four groups in the arm, and the four groups in the leg.
Apart from the contractile part of a muscle consisting of its fibers, a muscle contains a non-contractile part of dense fibrous connective tissue that makes up the tendon at each end. The tendons attach the muscles to bones to give skeletal movement. The length of a muscle includes the tendons. Connective tissue is present in all muscles as deep fascia. Deep fascia specialises within muscles to enclose each muscle fiber as endomysium; each muscle fascicle as perimysium, and each individual muscle as epimysium. Together these layers are called mysia. Deep fascia also separates the groups of muscles into muscle compartments.
Two types of
Skeletal muscle cells
Skeletal muscle cells are the individual contractile cells within a muscle, and are often termed as muscle fibers.[3] A single muscle such as the biceps in a young adult male contains around 253,000 muscle fibers.[22]
Skeletal muscle fibers are
Many nuclei are needed by the skeletal muscle cell for the large amounts of proteins and enzymes needed to be produced for the cell's normal functioning. A single muscle fiber can contain from hundreds to thousands of nuclei.[25] A muscle fiber for example in the human biceps with a length of 10 cm can have as many as 3,000 nuclei.[25] Unlike in a non-muscle cell where the nucleus is centrally positioned, the myonucleus is elongated and located close to the sarcolemma. The myonuclei are quite uniformly arranged along the fiber with each nucleus having its own myonuclear domain where it is responsible for supporting the volume of cytoplasm in that particular section of the myofiber.[24][25]
A group of muscle stem cells known as myosatellite cells, also satellite cells are found between the basement membrane and the sarcolemma of muscle fibers. These cells are normally quiescent but can be activated by exercise or pathology to provide additional myonuclei for muscle growth or repair.[26]
Attachment to tendons
Muscles attach to tendons in a complex interface region known as the musculotendinous junction, also known as the myotendinous junction, an area specialised for the primary transmission of force.[27] At the muscle-tendon interface, force is transmitted from the sarcomeres in the muscle cells to the tendon.[5] Muscles and tendons develop in close association, and after their joining at the myotendinous junction they constitute a dynamic unit for the transmission of force from muscle contraction to the skeletal system.[27]
Arrangement of muscle fibers

Muscle architecture refers to the arrangement of muscle fibers relative to the axis of force generation, which runs from a muscle's origin to its insertion. The usual arrangements are types of
The fibers in
Muscle fiber growth
Muscle fibers grow when exercised and shrink when not in use. This is due to the fact that exercise stimulates the increase in
Muscle naming
There are a number of terms used in the naming of muscles including those relating to size, shape, action, location, their orientation, and their number of heads.
- By size
- brevis means short; longus means long; longissimus means longest; magnus means large; major means larger; maximus means largest; minor means smaller, and minimus smallest; latissimus means widest, and vastus means huge.[31] These terms are often used after the particular muscle such as gluteus maximus, and gluteus minimus.[32]
- By relative shape
- deltoid means triangular; quadratus means having four sides; rhomboideus means having a pronator quadratus.
- By action
- fixator musclesserve to fix a joint in a given position by stabilizing the prime mover whilst other joints are moving.
- By number of heads
- biceps two; triceps three and quadriceps four.[32]
- By location
- named after the near main structure such as the temporal muscle (temporalis) near to the temporal bone.[31] Also supra- above; infra- below, and sub- under.[19]
- By fascicle orientation
- Relative to the midline, rectus means parallel to the midline; transverse means perpendicular to the midline, and oblique means diagonal to the midline.[31] Relative to the axis of the generation of force – types of parallel, and types of pennate muscles.
Fiber types
Broadly there are two types of muscle fiber: Type I, which is slow, and Type II which are fast. Type II has two divisions of type IIA (oxidative), and type IIX (glycolytic), giving three main fiber types.[33] These fibers have relatively distinct metabolic, contractile, and motor unit properties. The table below differentiates these types of properties. These types of properties—while they are partly dependent on the properties of individual fibers—tend to be relevant and measured at the level of the motor unit, rather than individual fiber.[34]
| Properties | Type I fibers | Type IIA fibers | Type IIX fibers |
|---|---|---|---|
| Motor Unit Type | Slow Oxidative (SO) | Fast Oxidative/Glycolytic (FOG) | Fast Glycolytic (FG) |
| Twitch speed | Slow | Fast | Fast |
| Twitch force | Small | Medium | Large |
| Resistance to fatigue | High | High | Low |
| Glycogen content | Low | High | High |
| Capillary supply | Rich | Rich | Poor |
| Capillary density | High | Intermediate | Low |
| Myoglobin | High | High | Low |
| Red color | Dark | Dark | Pale |
| Mitochondrial density | High | High | Low |
| Oxidative enzyme capacity | High | Intermediate-high | Low |
| Z-line width | Intermediate | Wide | Narrow |
| Alkaline ATPase activity | Low | High | High |
| Acidic ATPase activity | High | Medium-high | Low |
Slow oxidative (type I) fibers contract relatively slowly and use aerobic respiration to produce ATP. Fast oxidative (type IIA) fibers have fast contractions and primarily use aerobic respiration, but because they may switch to anaerobic respiration (glycolysis), can fatigue more quickly than slow oxidative fibers. Fast glycolytic (type IIX) fibers have fast contractions and primarily use anaerobic glycolysis. The FG fibers fatigue more quickly than the others. Most skeletal muscles in a human contain(s) all three types, although in varying proportions.[35]
Fiber color
Traditionally, fibers were categorized depending on their varying color, which is a reflection of
Twitch speed
Fibers can also be classified on their twitch capabilities, into fast and slow twitch. These traits largely, but not completely, overlap the classifications based on color, ATPase, or MHC (
Some authors define a fast twitch fiber as one in which the myosin can split ATP very quickly. These mainly include the ATPase type II and MHC type II fibers. However, fast twitch fibers also demonstrate a higher capability for electrochemical transmission of action potentials and a rapid level of calcium release and uptake by the sarcoplasmic reticulum. The fast twitch fibers rely on a well-developed, anaerobic, short term, glycolytic system for energy transfer and can contract and develop tension at 2–3 times the rate of slow twitch fibers. Fast twitch muscles are much better at generating short bursts of strength or speed than slow muscles, and so fatigue more quickly.[36]
The slow twitch fibers generate energy for ATP re-synthesis by means of a long term system of aerobic energy transfer. These mainly include the ATPase type I and MHC type I fibers. They tend to have a low activity level of ATPase, a slower speed of contraction with a less well developed glycolytic capacity.[36] Fibers that become slow-twitch develop greater numbers of mitochondria and capillaries making them better for prolonged work.[37]
Type distribution
Individual muscles tend to be a mixture of various fiber types, but their proportions vary depending on the actions of that muscle. For instance, in humans, the
The total number of skeletal muscle fibers has traditionally been thought not to change. It is believed there are no sex or age differences in fiber distribution; however, proportions of fiber types vary considerably from muscle to muscle and person to person.[citation needed] Among different species there is much variation in the proportions of muscle fiber types.[39]
Sedentary men and women (as well as young children) have 45% type II and 55% type I fibers.[citation needed] People at the higher end of any sport tend to demonstrate patterns of fiber distribution e.g. endurance athletes show a higher level of type I fibers. Sprint athletes, on the other hand, require large numbers of type IIX fibers. Middle-distance event athletes show approximately equal distribution of the two types. This is also often the case for power athletes such as throwers and jumpers. It has been suggested that various types of exercise can induce changes in the fibers of a skeletal muscle.[40]
It is thought that by performing endurance type events for a sustained period of time, some of the type IIX fibers transform into type IIA fibers. However, there is no consensus on the subject.[citation needed] It may well be that the type IIX fibers show enhancements of the oxidative capacity after high intensity endurance training which brings them to a level at which they are able to perform oxidative metabolism as effectively as slow twitch fibers of untrained subjects. This would be brought about by an increase in mitochondrial size and number and the associated related changes, not a change in fiber type.
Fiber typing methods

There are numerous methods employed for fiber-typing, and confusion between the methods is common among non-experts. Two commonly confused methods are
When "type I" or "type II" fibers are referred to generically, this most accurately refers to the sum of numerical fiber types (I vs. II) as assessed by myosin ATPase activity staining (e.g. "type II" fibers refers to type IIA + type IIAX + type IIXA ... etc.).
Below is a table showing the relationship between these two methods, limited to fiber types found in humans. Subtype capitalization is used in fiber typing vs. MHC typing, and some ATPase types actually contain multiple MHC types. Also, a subtype B or b is not expressed in humans by either method.[41] Early researchers believed humans to express a MHC IIb, which led to the ATPase classification of IIB. However, later research showed that the human MHC IIb was in fact IIx,[41] indicating that the IIB is better named IIX. IIb is expressed in other mammals, so is still accurately seen (along with IIB) in the literature. Non human fiber types include true IIb fibers, IIc, IId, etc.
| ATPase type | MHC heavy chain(s) |
|---|---|
| Type I | MHC Iβ |
| Type IC | MHC Iβ > MHC IIa |
| Type IIC | MHC IIa > MHC Iβ |
| Type IIA | MHC IIa |
| Type IIAX | MHC IIa > MHC IIx |
| Type IIXA | MHC IIx > MHC IIa |
| Type IIX | MHC IIx |
Further fiber typing methods are less formally delineated, and exist on more of a spectrum. They tend to be focused more on metabolic and functional capacities (i.e., oxidative vs. glycolytic, fast vs. slow contraction time). As noted above, fiber typing by ATPase or MHC does not directly measure or dictate these parameters. However, many of the various methods are mechanistically linked, while others are correlated in vivo.[44][45] For instance, ATPase fiber type is related to contraction speed, because high ATPase activity allows faster crossbridge cycling.[34] While ATPase activity is only one component of contraction speed, Type I fibers are "slow", in part, because they have low speeds of ATPase activity in comparison to Type II fibers. However, measuring contraction speed is not the same as ATPase fiber typing.
Muscle fiber type evolution
Almost all multicellular animals depend on muscles to move.[46] Generally, muscular systems of most multicellular animals comprise both slow-twitch and fast-twitch muscle fibers, though the proportions of each fiber type can vary across organisms and environments. The ability to shift their phenotypic fiber type proportions through training and responding to the environment has served organisms well when placed in changing environments either requiring short explosive movements (higher fast twitch proportion) or long duration of movement (higher slow twitch proportion) to survive.[47] Bodybuilding has shown that changes in muscle mass and force production can change in a matter of months.[48] Some examples of this variation are described below.[49]
Examples of muscle fiber variation in different animals
Invertebrates
American lobster, Homarus americanus, has three fiber types including fast twitch fibers, slow-twitch and slow-tonic fibers.[50] Slow-tonic is a slow twitch-fiber that can sustain longer contractions (tonic).[51][52] In lobsters, muscles in different body parts vary in the muscle fiber type proportions based on the purpose of the muscle group.[50]
Vertebrates
In the early
Reptiles
In larger animals, different muscle groups will increasingly require different fiber type proportions within muscle for different purposes. Turtles, such as Trachemys scripta elegans, have complementary muscles within the neck that show a potential inverse trend of fiber type percentages (one muscle has high percentage of fast twitch, while the complementary muscle will have a higher percentage of slow twitch fibers). The complementary muscles of turtles had similar percentages of fiber types.[51]
Mammals
Chimpanzee muscles are composed of 67% fast-twitch fibers and have a maximum dynamic force and power output 1.35 times higher than human muscles of similar size. Among mammals, there is a predominance of type II fibers utilizing glycolytic metabolism. Because of the discrepancy in fast twitch fibers compared to humans, chimpanzees outperform humans in power related tests. Humans, however, will do better at exercise in aerobic range requiring large metabolic costs such as walking (bipedalism).[54]
Genetic conservation versus functional conservation
Across species, certain gene sequences have been preserved, but do not always have the same functional purpose. Within the zebrafish embryo, the Prdm1 gene down-regulates the formation of new slow twitch fibers through direct and indirect mechanisms such as Sox6 (indirect). In mice, the Prdm1 gene is present but does not control slow muscle genes in mice through Sox6.[55]
Plasticity
In addition to having a genetic basis, the composition of muscle fiber types is flexible and can vary with a number of different environmental factors. This plasticity can, arguably, be the strongest evolutionary advantage among organisms with muscle.
In fish, different fiber types are expressed at different water temperatures.[53] Cold temperatures require more efficient metabolism within muscle and fatigue resistance is important. While in more tropical environments, fast powerful movements (from higher fast-twitch proportions) may prove more beneficial in the long run.[56]
In rodents such as rats, the transitory nature of their muscle is highly prevalent. They have high percentage of hybrid muscle fibers and have up to 60% in fast-to-slow transforming muscle.[48]
Environmental influences such as diet, exercise and lifestyle types have a pivotal role in proportions of fiber type in humans. Aerobic exercise will shift the proportions towards slow twitch fibers, while explosive powerlifting and sprinting will transition fibers towards fast twitch.[47] In animals, "exercise training" will look more like the need for long durations of movement or short explosive movements to escape predators or catch prey.[57]
Microanatomy
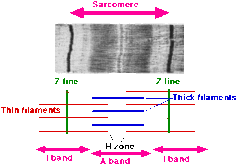
Skeletal muscle exhibits a distinctive banding pattern when viewed under the microscope due to the arrangement of two contractile proteins myosin, and actin – that are two of the myofilaments in the myofibrils. The myosin forms the thick filaments, and actin forms the thin filaments, and are arranged in repeating units called sarcomeres. The interaction of both proteins results in muscle contraction.
The sarcomere is attached to other organelles such as the mitochondria by intermediate filaments in the cytoskeleton. The costamere attaches the sarcomere to the sarcolemma.[5]
Every single organelle and macromolecule of a muscle fiber is arranged to ensure that it meets desired functions. The
While the muscle fiber does not have smooth endoplasmic cisternae, it contains
Development
All muscles are derived from
During development,
Between the tenth and the eighteenth weeks of gestation, all muscle cells have fast myosin heavy chains; two myotube types become distinguished in the developing fetus – both expressing fast chains but one expressing fast and slow chains. Between 10 and 40 per cent of the fibers express the slow myosin chain.[60]
Fiber types are established during embryonic development and are remodelled later in the adult by neural and hormonal influences.[39] The population of satellite cells present underneath the basal lamina is necessary for the postnatal development of muscle cells.[61]
Function
The primary function of muscle is
Muscle also functions to produce body heat. Muscle contraction is responsible for producing 85% of the body's heat.

Contraction

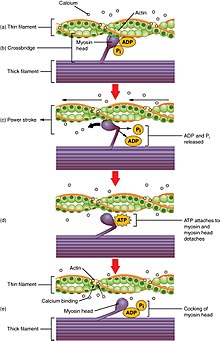
Contraction is achieved by the muscle's structural unit, the muscle fiber, and by its functional unit, the
In addition to the
Excitation-contraction coupling
back into the sarcoplasmic reticulum. As Ca2+
declines back to resting levels, the force declines and relaxation occurs.[68]
Muscle movement
The efferent leg of the peripheral nervous system is responsible for conveying commands to the muscles and glands, and is ultimately responsible for voluntary movement. Nerves move muscles in response to voluntary and autonomic (involuntary) signals from the brain. Deep muscles, superficial muscles, muscles of the face and internal muscles all correspond with dedicated regions in the primary motor cortex of the brain, directly anterior to the central sulcus that divides the frontal and parietal lobes.
In addition, muscles react to
Nerves that control skeletal muscles in
Deeper muscles such as those involved in
Proprioception
In skeletal muscles, muscle spindles convey information about the degree of muscle length and stretch to the central nervous system to assist in maintaining posture and joint position. The sense of where our bodies are in space is called proprioception, the perception of body awareness, the "unconscious" awareness of where the various regions of the body are located at any one time. Several areas in the brain coordinate movement and position with the feedback information gained from proprioception. The cerebellum and red nucleus in particular continuously sample position against movement and make minor corrections to assure smooth motion.[citation needed]
Energy consumption

Muscular activity accounts for much of the body's
Skeletal muscle uses more calories than other organs.[69] At rest it consumes 54.4 kJ/kg (13.0 kcal/kg) per day. This is larger than adipose tissue (fat) at 18.8 kJ/kg (4.5 kcal/kg), and bone at 9.6 kJ/kg (2.3 kcal/kg).[70]
Efficiency
The
Muscle strength
| Grade 0 | No contraction |
| Grade 1 | Trace of contraction, but no movement at the joint |
| Grade 2 | Movement at the joint with gravity eliminated |
| Grade 3 | Movement against gravity, but not against added resistance |
| Grade 4 | Movement against external resistance, but less than normal |
| Grade 5 | Normal strength |
Muscle strength is a result of three overlapping factors: physiological strength (muscle size, cross sectional area, available crossbridging, responses to training), neurological strength (how strong or weak is the signal that tells the muscle to contract), and mechanical strength (muscle's force angle on the lever, moment arm length, joint capabilities).[citation needed]
Vertebrate muscle typically produces approximately 25–33
The strength of any given muscle, in terms of force exerted on the skeleton, depends upon length, shortening speed, cross sectional area, pennation, sarcomere length, myosin isoforms, and neural activation of motor units. Significant reductions in muscle strength can indicate underlying pathology, with the chart at right used as a guide.
The maximum holding time for a contracted muscle depends on its supply of energy and is stated by Rohmert's law to exponentially decay from the beginning of exertion.
The "strongest" human muscle
This section needs additional citations for verification. (March 2016) |
Since three factors affect muscular strength simultaneously and muscles never work individually, it is misleading to compare strength in individual muscles, and state that one is the "strongest". But below are several muscles whose strength is noteworthy for different reasons.
- In ordinary parlance, muscular "strength" usually refers to the ability to exert a force on an external object—for example, lifting a weight. By this definition, the ) for 2 seconds. What distinguishes the masseter is not anything special about the muscle itself, but its advantage in working against a much shorter lever arm than other muscles.
- If "strength" refers to the force exerted by the muscle itself, e.g., on the place where it inserts into a bone, then the strongest muscles are those with the largest cross-sectional area. This is because the tension exerted by an individual skeletal muscle fiber does not vary much. Each fiber can exert a force on the order of 0.3 micronewton. By this definition, the strongest muscle of the body is usually said to be the quadriceps femoris or the gluteus maximus.
- Because muscle strength is determined by cross-sectional area, a shorter muscle will be stronger "pound for pound" (i.e., by myometrial layer of the uterus may be the strongest muscle by weight in the female body. At the time when an infantis delivered, the entire uterus weighs about 1.1 kg (40 oz). During childbirth, the uterus exerts 100 to 400 N (25 to 100 lbf) of downward force with each contraction.
- The external muscles of the eye are conspicuously large and strong in relation to the small size and weight of the eyeball. It is frequently said that they are "the strongest muscles for the job they have to do" and are sometimes claimed to be "100 times stronger than they need to be." However, eye movements (particularly saccades used on facial scanning and reading) do require high speed movements, and eye muscles are exercised nightly during rapid eye movement sleep.
- The statement that "the tongue is the strongest muscle in the body" appears frequently in lists of surprising facts, but it is difficult to find any definition of "strength" that would make this statement true. The tongue consists of eight muscles, not one.
Force generation
Contracting muscles produce vibration and sound.[74] Slow twitch fibers produce 10 to 30 contractions per second (10 to 30 Hz). Fast twitch fibers produce 30 to 70 contractions per second (30 to 70 Hz).[75] The vibration can be witnessed and felt by highly tensing one's muscles, as when making a firm fist. The sound can be heard by pressing a highly tensed muscle against the ear, again a firm fist is a good example. The sound is usually described as a rumbling sound. Some individuals can voluntarily produce this rumbling sound by contracting the tensor tympani muscle of the middle ear. The rumbling sound can also be heard when the neck or jaw muscles are highly tensed.[citation needed]
Signal transduction pathways
Skeletal muscle fiber-type phenotype in adult animals is regulated by several independent signaling pathways. These include pathways involved with the
Contraction-induced changes in intracellular calcium or reactive oxygen species provide signals to diverse pathways that include the MAPKs, calcineurin and calcium/calmodulin-dependent protein kinase IV to activate transcription factors that regulate gene expression and enzyme activity in skeletal muscle.

PGC1-α (
The transition from aerobic to anaerobic metabolism during intense work requires that several systems are rapidly activated to ensure a constant supply of ATP for the working muscles. These include a switch from fat-based to carbohydrate-based fuels, a redistribution of blood flow from nonworking to exercising muscles, and the removal of several of the by-products of anaerobic metabolism, such as carbon dioxide and lactic acid. Some of these responses are governed by transcriptional control of the fast twitch (FT) glycolytic phenotype. For example, skeletal muscle reprogramming from an ST glycolytic phenotype to an FT glycolytic phenotype involves the Six1/Eya1 complex, composed of members of the Six protein family. Moreover, the hypoxia-inducible factor 1-α (HIF1A) has been identified as a master regulator for the expression of genes involved in essential hypoxic responses that maintain ATP levels in cells. Ablation of HIF-1α in skeletal muscle was associated with an increase in the activity of rate-limiting enzymes of the mitochondria, indicating that the citric acid cycle and increased fatty acid oxidation may be compensating for decreased flow through the glycolytic pathway in these animals. However, hypoxia-mediated HIF-1α responses are also linked to the regulation of mitochondrial dysfunction through the formation of excessive reactive oxygen species in mitochondria.
Other pathways also influence adult muscle character. For example, physical force inside a muscle fiber may release the transcription factor serum response factor from the structural protein titin, leading to altered muscle growth.
Exercise

Physical exercise is often recommended as a means of improving motor skills, fitness, muscle and bone strength, and joint function. Exercise has several effects upon muscles, connective tissue, bone, and the nerves that stimulate the muscles. One such effect is muscle hypertrophy, an increase in size of muscle due to an increase in the number of muscle fibers or cross-sectional area of myofibrils.[76] Muscle changes depend on the type of exercise used.
Generally, there are two types of exercise regimes, aerobic and anaerobic.
The presence of
Clinical significance
Muscle disease
Diseases of skeletal muscle are termed

Neuromuscular diseases affect the muscles and their nervous control. In general, problems with nervous control can cause spasticity or paralysis, depending on the location and nature of the problem. A number of movement disorders are caused by neurological disorders such as Parkinson's disease and Huntington's disease where there is central nervous system dysfunction.[85]
Symptoms of muscle diseases may include weakness, spasticity, myoclonus and myalgia. Diagnostic procedures that may reveal muscular disorders include testing creatine kinase levels in the blood and electromyography (measuring electrical activity in muscles). In some cases, muscle biopsy may be done to identify a myopathy, as well as genetic testing to identify DNA abnormalities associated with specific myopathies and dystrophies.
A non-invasive
Hypertrophy
Independent of strength and performance measures, muscles can be induced to grow larger by a number of factors, including hormone signaling, developmental factors,
Biological factors such as age and hormone levels can affect muscle hypertrophy. During puberty in males, hypertrophy occurs at an accelerated rate as the levels of growth-stimulating hormones produced by the body increase. Natural hypertrophy normally stops at full growth in the late teens. As testosterone is one of the body's major growth hormones, on average, men find hypertrophy much easier to achieve than women. Taking additional testosterone or other anabolic steroids will increase muscular hypertrophy.
Muscular, spinal and neural factors all affect muscle building. Sometimes a person may notice an increase in strength in a given muscle even though only its opposite has been subject to exercise, such as when a bodybuilder finds her left biceps stronger after completing a regimen focusing only on the right biceps. This phenomenon is called cross education.[citation needed]
Atrophy

Every day between one and two percent of muscle is broken down and rebuilt.
Human spaceflight, involving prolonged periods of immobilization and weightlessness is known to result in muscle weakening and atrophy resulting in a loss of as much as 30% of mass in some muscles.[90][91] Such consequences are also noted in some mammals following hibernation.[92]
Many diseases and conditions including
Research
Myopathies have been modeled with cell culture systems of muscle from healthy or diseased tissue
Research on skeletal muscle properties uses many techniques. Electrical muscle stimulation is used to determine force and contraction speed at different frequencies related to fiber-type composition and mix within an individual muscle group. In vitro muscle testing is used for more complete characterization of muscle properties.
The electrical activity associated with muscle contraction is measured via
Research into the development of artificial muscles includes the use of electroactive polymers.

Mononuclear cells of skeletal muscle
Nuclei present in skeletal muscle are about 50% myocyte nuclei and 50% mononuclear cell nuclei.
Endocrine functions of skeletal muscle
As pointed out in the Introduction to this article, under different physiological conditions, subsets of 654 different proteins as well as lipids, amino acids, metabolites and small RNAs occur in the secretome of skeletal muscles.[12] As described in the Wikipedia article "List of human endocrine organs and actions", skeletal muscle is identified as an endocrine organ due to its secretion of cytokines and other peptides produced by skeletal muscle as signaling molecules. Iizuka et al.,[9] indicated that skeletal muscle is an endocrine organ because it "synthesizes and secretes multiple factors, and these muscle derived-factors exert beneficial effects on peripheral and remote organs." The altered secretomes after endurance training or resistance training as well as the secretome of sedentary muscle appear to have many effects on distant tissues.
Sedentary skeletal muscle mass affects executive mental function
A study in Canada tested the effect of muscle mass on mental functions during aging. An expectation of the study was that the endocrine components of the secretome specific to skeletal muscle could protect cognitive functions. The skeletal muscle mass of arms and legs of 8,279 Canadians over the age of 65 and in average health was measured at baseline and after three years.[103] Of these individuals, 1,605 participants (19.4%) were considered to have a low skeletal muscle mass at baseline, with less than 7.30 kg/m2 for males, and less than 5.42 kg/m2 for females (levels defined as sarcopenia in Canada).
Walking, using skeletal muscles, affects mortality
Paluch et al.[104] compared the average number of steps walked per day to the risk of mortality, both for adults over 60 years old and for adults under 60 years old. The study was a meta-analysis of 15 studies, which, combined, evaluated 47,471 adults over a period of 7 years. Individuals were divided into approximately equal quartiles. The lowest quartile averaged 3,553 steps/day, the second quartile 5,801 steps/day, the third quartile 7,842 steps/day and the fourth quartile 10,901 steps/day. The briskness of walking, adjusted for the volume of walking, did not affect mortality. However, the number of steps/day was clearly related to mortality. When risk of mortality for those over 60 years old was set at 1.0 for the lowest quartile of steps/day, the relative risk of mortality for the second, third and fourth quartiles were 0.56, 0.45, and 0.35, respectively. For those under 60 years of age, the results were less pronounced. For those under 60 years of age, with the first quartile risk of mortality set at 1.0, the second, third and fourth quartile relative risks of mortality were 0.57, 0.42 and 0.53, respectively. Thus, use of skeletal muscles in walking has a large effect, especially among older individuals, on mortality.
Skeletal muscle secretome alters with exercise
Williams et al.[102] obtained biopsies of a thigh skeletal muscle (vastus lateralis muscle) of eight 23-year old, originally sedentary, Caucasian males. Biopsies were taken both before and after a six-week long endurance exercise training program. The exercise consisted of riding a stationary bicycle for one hour, five days a week for six weeks.
Of the 13,108 genes with detected expression in the muscle biopsies, 641 genes were upregulated after endurance training and 176 genes were downregulated. Of the 817 total altered genes, 531 were identified as being in the secretome by either or both of
Exercise-trained effects are mediated by epigenetic mechanisms
Between 2012 and 2019, at least 25 reports indicated a major role of
Exercise-induced regulation of genes in muscles

Gene expression in muscle is largely regulated, as in tissues generally, by regulatory DNA sequences, especially enhancers. Enhancers are non-coding sequences in the genome that activate the expression of distant target genes,[107] by looping around and interacting with the promoters of their target genes[108] (see Figure "Regulation of transcription in mammals"). As reported by Williams et al.,[102] the average distance in the loop between the connected enhancers and promoters of genes is 239,000 nucleotide bases.
Exercise-induced alteration to gene expression by DNA methylation or demethylation
Endurance muscle training alters muscle gene expression by epigenetic DNA methylation or de-methylation of CpG sites within enhancers.[109]
In a study by Lindholm et al.,[109] twenty-three individuals who were about 27 years old and sedentary volunteered to have endurance training on only one leg during 3 months. The other leg was used as an untrained control leg. The training consisted of one-legged knee extension training for 3 month (45 min, 4 sessions per week). Skeletal muscle biopsies from the vastus lateralis (a thigh muscle) were taken both before training began and 24 hours after the last training session from each of the legs. The endurance-trained leg, compared to the untrained leg, had significant DNA methylation changes at 4,919 sites across the genome. The sites of altered DNA methylation were predominantly in enhancers. Transcriptional analysis, using RNA sequencing, identified 4,076 differentially expressed genes.
The transcriptionally upregulated genes were associated with enhancers that had a significant decrease in DNA methylation, while transcriptionally downregulated genes were associated with enhancers that had increased DNA methylation. Increased methylation was mainly associated with genes involved in structural remodeling of the muscle and glucose metabolism. Enhancers with decreased methylation were associated with genes functioning in inflammatory or immunological processes and in transcriptional regulation.
Exercise-induced long-term alteration of gene expression by histone acetylation or deacetylation
As indicated above, after exercise, epigenetic alterations to enhancers alter long-term expression of hundreds of muscle genes.[102] This includes genes producing proteins secreted into the systemic circulation, many of which may act as endocrine messengers.[102] Six sedentary, about 23 years old, Caucasian males provided vastus lateralis (a thigh muscle) biopsies before entering an exercise program (six weeks of 60-minute sessions of riding a stationary cycle, five days per week). Four days after this exercise program was completed, the expression of many genes was persistently epigentically altered. The alterations altered acetylations and deacetylations of the histone tails located in the enhancers controlling the genes with altered expression.[102]
Up-regulated genes were associated with epigenetic acetylations added at histone 3 lysine 27 (H3K27ac) of nucleosomes located at their enhancers. Down-regulated genes were associated with the removal of epigenetic acetylations at H3K27 in nucleosomes located at their enhancers (see Figure "A nucleosome with histone tails set for transcriptional activation"). Biopsies of the vastus lateralis muscle showed expression of 13,108 genes at baseline before the exercise training program. Four days after the exercise program was completed, biopsies of the same muscles showed altered gene expression, with 641 genes up-regulated and 176 genes down-regulated.[102] Williams et al. identified 599 enhancer-gene interactions, covering 491 enhancers and 268 genes (multiple enhancers were found connected to some genes), where both the enhancer and the connected target gene were coordinately either upregulated or downregulated after exercise training.[102]
See also
References
- PMID 23517218.
- ^ Betts, J. Gordon; Young, Kelly A.; Wise, James A.; Johnson, Eddie; Poe, Brandon; Kruse, Dean H.; Korol, Oksana; Johnson, Jody E.; Womble, Mark; Desaix, Peter (6 March 2013). "Interactions of Skeletal Muscles, Their Fascicle Arrangement, and Their Lever Systems". Interactions of skeletal muscles. OpenStax. Archived from the original on 23 March 2022. Retrieved 24 May 2021.
- ^ a b c "Structure of Skeletal Muscle | SEER Training". training.seer.cancer.gov.
- ^ ISBN 9781496347213.
- ^ PMID 28640448.
- ^ Brainard, Jean; Gray-Wilson, Niamh; Harwood, Jessica; Karasov, Corliss; Kraus, Dors; Willan, Jane (2011). CK-12 Life Science Honors for Middle School. CK-12 Foundation. p. 451. Retrieved 18 April 2015.
- S2CID 9232367.
- – via StatPearls [Internet].
- ^ PMID 24859778.
- PMID 28389517.
- PMID 32393961.
- ^ PMID 36474563.
- ^ PMID 32281477.
- ^ Alberts, Bruce; Johnson, Alexander; Lewis, Julian; Raff, Martin; Roberts, Keith; Walter, Peter (2002). Genesis, Modulation, and Regeneration of Skeletal Muscle. Garland Science.
- ^ PMID 30922843.
- ^ Introduction. Morgan & Claypool Life Sciences. 2011.
- PMID 33085295.
- ISBN 978-1-107-66901-7. Retrieved 17 February 2023.
- ^ a b "Muscle Groups | SEER Training". training.seer.cancer.gov. Retrieved 17 May 2021.
- ^ "What is the strongest muscle in the human body?". Library of Congress. Retrieved 17 May 2021.
- ISBN 9780674967953. Retrieved 17 February 2023.
- S2CID 20508198.
- PMID 29898810.
- ^ S2CID 234362466.
- ^ PMID 32175681.
- PMID 28631758.
- ^ PMID 23738275.
- ^ ISBN 978-0-321-50042-7.
- ^ a b c d Lieber, Richard L. (2002) Skeletal muscle structure, function, and plasticity. Wolters Kluwer Health.
- ^ Ziser, Stephen. "&Muscle Cell Anatomy & Function" (PDF). www.austincc.edu. Archived (PDF) from the original on 23 September 2015. Retrieved 12 February 2015.
- ^ ISBN 9780470646083.
- ^ ISBN 9780071222075.
- ^ Betts, J. Gordon; Young, Kelly A.; Wise, James A.; Johnson, Eddie; Poe, Brandon; Kruse, Dean H.; Korol, Oksana; Johnson, Jody E.; Womble, Mark; Desaix, Peter (6 March 2013). Types of muscle fibers. OpenStax. Retrieved 17 June 2021.
- ^ ISBN 978-0-7360-4517-9.
- ISBN 978-1-947172-04-3.
- ^ a b "Muscle fiber type". About.com. Sports Medicine. Archived from the original on 21 November 2007. Retrieved 27 November 2007.
- ISBN 978-0-7360-4517-9.
- ^ PMID 4120482.
- ^ PMID 22013216.
- ISBN 978-1-930546-78-3.
- ^ PMID 7545970.
- S2CID 7820419.
- PMID 8281747.
- PMID 5472111.
- PMID 430414.[permanent dead link]
- ^ Magazine, Viviane Callier, Quanta. "Too Small for Big Muscles, Tiny Animals Use Springs". Scientific American. Retrieved 13 December 2022.
{{cite web}}: CS1 maint: multiple names: authors list (link) - ^ S2CID 9613549.
- ^ ISSN 8750-7587.
- S2CID 7024153.
- ^ a b "Fiber polymorphism in skeletal muscles of the American lobster, Homarus americanus: continuum between slow-twitch (S1) and slow-tonic (S2) fibers". journals.biologists.com. Retrieved 13 December 2022.
- ^ S2CID 36961922.
- PMID 17486578.
- ^ a b "Expression of multiple slow myosin heavy chain genes reveals a diversity of zebrafish slow twitch muscle fibres with differing requirements for Hedgehog and Prdm1 activity". journals.biologists.com. Retrieved 13 December 2022.
- PMID 28652350.
- ^ "Non Conservation of Function for the Evolutionarily Conserved Prdm1 Protein in the Control of the Slow Twitch Myogenic Program in the Mouse Embryo". academic.oup.com. Retrieved 13 December 2022.
- PMID 33251280.
- ^ McDougall, Christopher (2009). Born to Run: a hidden tribe, superathletes, and the Greatest Race Never Seen.
- ISBN 9780072943689.
- ^ a b c Sweeney, Lauren (1997). Basic Concepts in Embryology: A Student's Survival Guide (1st Paperback ed.). McGraw-Hill Professional.
- ISBN 978-0-7360-4517-9.
- PMID 29212890.
- PMID 29322779.
- PMID 23897689.
- PMID 30761018.
- ^ "Introduction to the Muscular System | SEER Training". training.seer.cancer.gov.
- ^ "1.5 Homeostasis - Anatomy and Physiology | OpenStax". openstax.org. 25 April 2013. Retrieved 25 June 2021.
- ISBN 0-7216-9549-3.
- PMID 28509964.
- PMID 29925809.
- S2CID 10257328.
- ISBN 978-0-7360-7966-2.
- ^ Quoted from National Skeletal Muscle Research Center; UCSD, Muscle Physiology Home Page – Skeletal Muscle Architecture Archived 18 July 2014 at the Wayback Machine, Effect of Muscle Architecture on Muscle Function
- ^ "9.6 Forces and Torques in Muscles and Joints - College Physics | OpenStax". openstax.org. 21 June 2012. Retrieved 15 May 2021.
- PMID 1541245.
- ^ [1], Peak Performance – Endurance training: understanding your slow twitch muscle fibers will boost performance
- S2CID 29191826.
- PMID 144412.
- PMID 4403464.
- PMID 6682735.
- PMID 635587.
- PMID 6036801.
- PMID 11579166.
- S2CID 2745168.
- S2CID 27583398.
- ^ "Overview of Movement Disorders - Brain, Spinal Cord, and Nerve Disorders". MSD Manual Consumer Version. Retrieved 24 June 2021.
- ^ Dumé, Belle (18 May 2007). "'Muscle noise' could reveal diseases' progression". NewScientist.com news service.
- ISBN 978-0-87044-621-4.
- S2CID 208792618.
- PMID 17904252.
- S2CID 44574997.
- ^ "NASA Muscle Atrophy Research (MARES) Website". Archived from the original on 4 May 2010.
- PMID 17307375.
- S2CID 42148927.[permanent dead link]
- S2CID 21241434.

- S2CID 34744926.
- S2CID 25169061.
- PMID 24622330.
- ^ S2CID 253383236.
- PMID 31937892.
- PMID 31496956.
- ^ "The skeletal muscle-specific proteome". The Human Protein Atlas.
- ^ PMID 34252634.
- ^ PMID 35796211.
- PMID 35247352.
- S2CID 73481438.
- PMID 24560929.
- PMID 33858480.
- PMID 23124110.
- ^ PMID 25484259.


