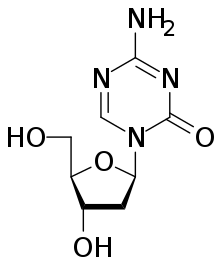
Decitabine
 | |
| Clinical data | |
|---|---|
| Trade names | Dacogen, Demylocan |
| Other names | 5-aza-2'-deoxycytidine |
| AHFS/Drugs.com | Monograph |
| MedlinePlus | a608009 |
| License data |
|
| Routes of administration | Intravenous |
| ATC code | |
| Legal status | |
| Legal status | |
| Pharmacokinetic data | |
| Protein binding | <1% |
| Elimination half-life | 30 minutes |
| Identifiers | |
| |
JSmol) | |
| |
| |
| | |
Decitabine (i.e., 5-aza-2′-deoxycytidine), sold under the brand name Dacogen among others, acts as a nucleic acid synthesis inhibitor.
Medical uses
Decitabine is used to treat
It also has EU approval for acute myeloid leukemia (AML).[4]
Pharmacology
This section needs additional citations for verification. (December 2018) |
Decitabine is a hypomethylating agent.[6][7] It hypomethylates DNA by inhibiting DNA methyltransferase.
It functions in a similar manner to azacitidine, although decitabine can only be incorporated into DNA strands while azacitidine can be incorporated into both DNA and RNA chains.
It incorporates into DNA strands upon replication, and then when DNA methyltransferases (DNMTs) such as DNMT1, are engaged to bind the DNA and to replicate the methylation to the daughter strand, DNMTs are bound to decitabine irreversibly and cannot disengage. Therefore, the action of decitabine is division-dependent, meaning the cells have to divide in order for the pharmaceutical to act. Therefore, cancer cells which divide much more rapidly than most other cells in the body will be more severely affected by decitabine just because they replicate more. It seems that DNA hypermethylation is critical for development of cancer cells, and specifically for haematological malignancies. Methylation of CpG islands upstream of tumor suppressor genes in order to silence them seems to be critical for these type of cancers. Thus at optimal doses, decitabine blocks this type of methylation and has an anti-neoplastic effect.
Research
Atherosclerosis
A number of investigators have shown a relationship between
References
- ^ "Summary Basis of Decision (SBD) for Dacogen". Health Canada. 23 October 2014. Retrieved 29 May 2022.
- ^ "Summary Basis of Decision (SBD) for Demylocan". Health Canada. 23 October 2014. Retrieved 29 May 2022.
- ^ "Decitabine". National Center for Biotechnology Information. Retrieved September 24, 2016.
- ^ a b "EC Approves Marketing Authorization Of DACOGEN For Acute Myeloid Leukemia". 2012-09-28. Retrieved 28 September 2012.
- PMID 20511166.
- S2CID 9556660.
- S2CID 1149318.
- PMID 25953647.
Further reading
- Moon C, Kim SH, Park KS, Choi BK, Lee HS, Park JB, et al. (June 2009). "Use of epigenetic modification to induce FOXP3 expression in naïve T cells". Transplantation Proceedings. 41 (5): 1848–1854. PMID 19545742.
External links
- "Decitabine". Drug Information Portal. U.S. National Library of Medicine.
