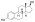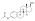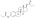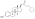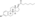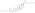Estradiol dienantate
 | |
 | |
| Clinical data | |
|---|---|
| Trade names | Climacteron, Amenose, Lactimex, Lactostat (all combinations) |
| Other names | Estradiol dienantate; EDE; EDEn; E2-EDN; Estradiol diheptanoate; Estra-1,3,5(10)-triene-3,17β-diol 3,17β-diheptanoate |
| Routes of administration | Intramuscular injection |
| Drug class | Estrogen; Estrogen ester |
| Identifiers | |
| |
JSmol) | |
| |
| |
Estradiol dienanthate (EDE), sold under the brand names Climacteron among others, is a long-acting
EDE was first described by 1959.[12][13] It was previously available in Canada and Germany but was discontinued by 2005.[14][15][16] The medication is no longer available in any form.[5]
Medical uses
EDE, a long-acting estrogen, was used in combination with EB, a short-acting estrogen, and TEBH, a long-acting androgen/anabolic steroid, in
Available forms
EDE was available only in combination EB and TEBH.[17] The combination was available in two different dose forms, one for menopausal hormone therapy (brand names Climacteron, Amenose) and the other for lactation suppression (brand names Lactimex, Lactostat). Climacteron and Amenose contained 1.0 mg EB, 7.5 mg EDE, and 150 mg TEBH (69 mg free testosterone) and was given by repeated intramuscular injection at regular intervals.[6][18][8] Lactimex and Lactostat contained 6 mg EB, 15 mg EDE, and 300 mg TEBH in 2 mL of corn oil and was administered as a single intramuscular injection after childbirth or during breastfeeding.[7][19][20][21]
Pharmacology

Pharmacodynamics
EDE is an
| Estrogen | Form | Dose (mg) | Duration by dose (mg) | ||
|---|---|---|---|---|---|
| EPD | CICD | ||||
| Estradiol | Aq. soln. | ? | – | <1 d | |
| Oil soln. | 40–60 | – | 1–2 ≈ 1–2 d | ||
| Aq. susp. | ? | 3.5 | 0.5–2 ≈ 2–7 d; 3.5 ≈ >5 d | ||
| Microsph. | ? | – | 1 ≈ 30 d | ||
| Estradiol benzoate | Oil soln. | 25–35 | – | 1.66 ≈ 2–3 d; 5 ≈ 3–6 d | |
| Aq. susp. | 20 | – | 10 ≈ 16–21 d | ||
| Emulsion | ? | – | 10 ≈ 14–21 d | ||
| Estradiol dipropionate | Oil soln. | 25–30 | – | 5 ≈ 5–8 d | |
| Estradiol valerate | Oil soln. | 20–30 | 5 | 5 ≈ 7–8 d; 10 ≈ 10–14 d; 40 ≈ 14–21 d; 100 ≈ 21–28 d | |
| Estradiol benz. butyrate | Oil soln. | ? | 10 | 10 ≈ 21 d | |
| Estradiol cypionate | Oil soln. | 20–30 | – | 5 ≈ 11–14 d | |
| Aq. susp. | ? | 5 | 5 ≈ 14–24 d | ||
Estradiol enanthate |
Oil soln. | ? | 5–10 | 10 ≈ 20–30 d | |
| Estradiol dienanthate | Oil soln. | ? | – | 7.5 ≈ >40 d | |
| Estradiol undecylate | Oil soln. | ? | – | 10–20 ≈ 40–60 d; 25–50 ≈ 60–120 d | |
| Polyestradiol phosphate | Aq. soln. | 40–60 | – | 40 ≈ 30 d; 80 ≈ 60 d; 160 ≈ 120 d | |
| Estrone | Oil soln. | ? | – | 1–2 ≈ 2–3 d | |
| Aq. susp. | ? | – | 0.1–2 ≈ 2–7 d | ||
| Estriol | Oil soln. | ? | – | 1–2 ≈ 1–4 d | |
| Polyestriol phosphate | Aq. soln. | ? | – | 50 ≈ 30 d; 80 ≈ 60 d | |
Notes and sources
Notes: All ovulation-inhibiting dose of estradiol undecylate is 20–30 mg/month. Sources: See template. | |||||
-
Estradiol and testosterone levels after an intramuscular injection of 1 mgtestosterone enanthate benzilic acid hydrazone in oil (brand name Climacteron) in ovariectomized women.[22] Assays were performed using immunoassays.[22] Source was Sherwin (1987).[22]
Pharmacokinetics
Estradiol and testosterone levels following a single intramuscular injection of Climacteron (including 1 mg EB, 7.5 mg EDE, and 150 mg TEBH equivalent to 69 mg free testosterone) versus 10 mg estradiol valerate have been studied over 28 days.[23][24]
| Estrogen | Form | Dose (mg) | Duration by dose (mg) | ||
|---|---|---|---|---|---|
| EPD | CICD | ||||
| Estradiol | Aq. soln. | ? | – | <1 d | |
| Oil soln. | 40–60 | – | 1–2 ≈ 1–2 d | ||
| Aq. susp. | ? | 3.5 | 0.5–2 ≈ 2–7 d; 3.5 ≈ >5 d | ||
| Microsph. | ? | – | 1 ≈ 30 d | ||
| Estradiol benzoate | Oil soln. | 25–35 | – | 1.66 ≈ 2–3 d; 5 ≈ 3–6 d | |
| Aq. susp. | 20 | – | 10 ≈ 16–21 d | ||
| Emulsion | ? | – | 10 ≈ 14–21 d | ||
| Estradiol dipropionate | Oil soln. | 25–30 | – | 5 ≈ 5–8 d | |
| Estradiol valerate | Oil soln. | 20–30 | 5 | 5 ≈ 7–8 d; 10 ≈ 10–14 d; 40 ≈ 14–21 d; 100 ≈ 21–28 d | |
| Estradiol benz. butyrate | Oil soln. | ? | 10 | 10 ≈ 21 d | |
| Estradiol cypionate | Oil soln. | 20–30 | – | 5 ≈ 11–14 d | |
| Aq. susp. | ? | 5 | 5 ≈ 14–24 d | ||
Estradiol enanthate |
Oil soln. | ? | 5–10 | 10 ≈ 20–30 d | |
| Estradiol dienanthate | Oil soln. | ? | – | 7.5 ≈ >40 d | |
| Estradiol undecylate | Oil soln. | ? | – | 10–20 ≈ 40–60 d; 25–50 ≈ 60–120 d | |
| Polyestradiol phosphate | Aq. soln. | 40–60 | – | 40 ≈ 30 d; 80 ≈ 60 d; 160 ≈ 120 d | |
| Estrone | Oil soln. | ? | – | 1–2 ≈ 2–3 d | |
| Aq. susp. | ? | – | 0.1–2 ≈ 2–7 d | ||
| Estriol | Oil soln. | ? | – | 1–2 ≈ 1–4 d | |
| Polyestriol phosphate | Aq. soln. | ? | – | 50 ≈ 30 d; 80 ≈ 60 d | |
Notes and sources
Notes: All ovulation-inhibiting dose of estradiol undecylate is 20–30 mg/month. Sources: See template. | |||||
Chemistry
EDE is a
| Estrogen | Structure | Ester(s) | Relative mol. weight |
Relative E2 contentb |
log Pc | ||||
|---|---|---|---|---|---|---|---|---|---|
| Position(s) | Moiet(ies) | Type | Lengtha | ||||||
| Estradiol | – | – | – | – | 1.00 | 1.00 | 4.0 | ||
| Estradiol acetate | C3 | Ethanoic acid |
Straight-chain fatty acid | 2 | 1.15 | 0.87 | 4.2 | ||
| Estradiol benzoate | C3 | Benzoic acid | Aromatic fatty acid | – (~4–5) | 1.38 | 0.72 | 4.7 | ||
| Estradiol dipropionate | C3, C17β | Propanoic acid (×2) |
Straight-chain fatty acid | 3 (×2) | 1.41 | 0.71 | 4.9 | ||
| Estradiol valerate | C17β | Pentanoic acid |
Straight-chain fatty acid | 5 | 1.31 | 0.76 | 5.6–6.3 | ||
| Estradiol benzoate butyrate | C3, C17β | Benzoic acid, butyric acid | Mixed fatty acid | – (~6, 2) | 1.64 | 0.61 | 6.3 | ||
| Estradiol cypionate | C17β | Cyclopentylpropanoic acid |
Cyclic fatty acid | – (~6) | 1.46 | 0.69 | 6.9 | ||
Estradiol enanthate |
C17β | Heptanoic acid |
Straight-chain fatty acid | 7 | 1.41 | 0.71 | 6.7–7.3 | ||
| Estradiol dienanthate | C3, C17β | Heptanoic acid (×2) |
Straight-chain fatty acid | 7 (×2) | 1.82 | 0.55 | 8.1–10.4 | ||
| Estradiol undecylate | C17β | Undecanoic acid |
Straight-chain fatty acid | 11 | 1.62 | 0.62 | 9.2–9.8 | ||
| Estradiol stearate | C17β | Octadecanoic acid |
Straight-chain fatty acid | 18 | 1.98 | 0.51 | 12.2–12.4 | ||
| Estradiol distearate | C3, C17β | Octadecanoic acid (×2) |
Straight-chain fatty acid | 18 (×2) | 2.96 | 0.34 | 20.2 | ||
| Estradiol sulfate | C3 | Sulfuric acid | Water-soluble conjugate | – | 1.29 | 0.77 | 0.3–3.8 | ||
| Estradiol glucuronide | C17β | Glucuronic acid | Water-soluble conjugate | – | 1.65 | 0.61 | 2.1–2.7 | ||
| Estramustine phosphated | C3, C17β | Normustine, phosphoric acid | Water-soluble conjugate | – | 1.91 | 0.52 | 2.9–5.0 | ||
| Polyestradiol phosphatee | C3–C17β | Phosphoric acid | Water-soluble conjugate | – | 1.23f | 0.81f | 2.9g | ||
| Footnotes: a = Length of hydrophobicity). Retrieved from PubChem, ChemSpider, and DrugBank. d = Also known as estradiol normustine phosphate. e = Polymer of estradiol phosphate (~13 repeat units ). f = Relative molecular weight or estradiol content per repeat unit. g = log P of repeat unit (i.e., estradiol phosphate). Sources: See individual articles.
| |||||||||
History
EDE was first described and introduced for medical use by 1959.[12][13]
Society and culture
Brand names
EDE was marketed in combination with EB and TEBH under the brand names Climacteron, Amenose, Lactimex, and Lactostat.[17][8]
Availability
EDE is no longer available but was previously used in Canada, Germany and other countries.[5][17][7][21]
See also
References
- ^ ISBN 978-1-4757-2085-3.
- ^ ISBN 978-1-61737-129-5.
- ^ PMID 13901504.
- ^ ISBN 978-1-59259-246-3.
- ^ a b c "Estradiol: Uses, Dosage & Side Effects". Drugs.com.
- ^ a b c "Climacteron Drug Information, Professional". Drugs.com. Archived from the original on 2 June 2019. Retrieved 2 June 2019.
- ^ a b c d Geburtshilfe und Frauenheilkunde: Ergebnisse der Forschung für die Praxis. Georg Thieme Verlag. 1969. p. 387,390.
[Kelly and Primose and Dodek found the following androgen-estrogen combination to be particularly effective and well-tolerated: 300 mg 3-benzilic acid hydrazone-testosterone-17-enanthate, 15 mg estradiol di-enanthate, 6 mg estradiol benzoate in 2 ml corn oil. This product is sold in Germany under the name Lactimex and has been clinically examined by us.] [...] Of 1200 postpartum patients one quarter stopped breast feeding for a variety of reasons and received an injection of Lactimex (Protina: Benzil acid hydrazon-testosteron-oenanthat 300 mg, Oestradiol-di-oenanthat 15 mg and Oestradiol-benzoate 6 mg in 1.0 ml of oil). In 76% of cases one injection was sufficient and the remaining 24% required a second injection. A second injection was required rarer if the first injection had been longer after delivery. A higher dosage of Lactimex was not necessary in cases with a preceding medical induction with intraveinous Oxytocin (Orasthin). Mothers who had been treated postpartum with methylergobasin did not as often require a second injection. No localized or generalized adverse reaction to the drug was noticed.
- ^ ISBN 9783871930133.
49035 В Amenose® Rp Ampullen Zus.: 1 Amp. 1 ml enth.: Benzilsäurehydrazid-N-testosteron-hydrazon-17-oenanthat 150 mg, Oestradiol-di-oenanthat 7.5 mg. Oestradiolbenzoat 1 mg in öl-Lösg. Ind.: Androgen-Oestrogen-Gemisch. Gegen Ausfallserscheinungen im Klimakterium und nach Ovarektomie. Osteoporose. Kontraind.: A 90, О 5 Dos.: Durchschnittl. alle 6 Wochen 1 Amp. im. 1 Amp. I ml 6.75 3 Amp 17.40 AP.: 10 Amp.
- ISBN 978-0-19-975569-1.
- ^ S2CID 24616324.
- ^ ISBN 978-3-642-60107-1.
Natural estrogens considered here include: [...] Esters of 17β-estradiol, such as estradiol valerate, estradiol benzoate and estradiol cypionate. Esterification aims at either better absorption after oral administration or a sustained release from the depot after intramuscular administration. During absorption, the esters are cleaved by endogenous esterases and the pharmacologically active 17β-estradiol is released; therefore, the esters are considered as natural estrogens.
- ^ a b Vademecum International. J. Morgan Jones Publications. 1959. pp. 121–122.
- ^ PMID 13752392.
- PMID 22971455.
- .
- ^ "Discontinuation of CLIMACTERON® Injection (estradiol dienanthate ⁄ estradiol benzoate and testosterone enanthate benzilic acid hydrazone injection in corn oil)" (PDF). Sandoz. Health Canada. 23 November 2005. Archived from the original (PDF) on 2013-01-11. Retrieved 2 June 2019.
- ^ a b c "MDX Pharmaceutical Knowledge". Merative US L.P.
- S2CID 24695692.
- ^ Zentralblatt für Gynäkologie. J. A. Barth. 1971.
The preparation Lactimex (300 mg 3-benzyl hydrazone-testosterone-17-enanthate + 15 mg estradiol-dienanthate + 6 mg estradiol benzoate in 2 ml corn oil) was injected. [...]
- ISBN 978-0-323-15726-1.
- ^ ISBN 978-0-919115-04-0.
LACTOSTAT [...] Each 2 mL of injectable solution contains testosteorne enanthate benzilic acid hydrazone 300 mg, estradiol dienanthate 15 mg, estradiol benzoate 6 mg, benzyl alcohol 7.5% as preservative, benzyl benzoate 0.75 mg, corn oil q.s. Available in 2 mL ampuls, boxes of 25.
- ^ ISSN 0167-482X.
- ^ PMID 3826177.
- ^ PMID 2966832.

![Estradiol and testosterone levels after an intramuscular injection of 1 mg estradiol benzoate, 7.5 mg estradiol dienanthate, and 150 mg testosterone enanthate benzilic acid hydrazone in oil (brand name Climacteron) in ovariectomized women.[22] Assays were performed using immunoassays.[22] Source was Sherwin (1987).[22]](http://upload.wikimedia.org/wikipedia/commons/thumb/7/74/Estradiol_and_testosterone_levels_after_an_intramuscular_injection_of_Climacteron_in_women.png/450px-Estradiol_and_testosterone_levels_after_an_intramuscular_injection_of_Climacteron_in_women.png)
![Estradiol levels after an intramuscular injection of 10 mg estradiol valerate in oil or Climacteron (1 mg estradiol benzoate, 7.5 mg estradiol dienanthate in oil) in ovariectomized women.[23][24] Assays were performed using RIA.[23][24] Source was Sherwin et al. (1987).[23][24]](http://upload.wikimedia.org/wikipedia/commons/thumb/e/e5/Estradiol_levels_after_an_intramuscular_injection_of_Climacteron_or_estradiol_valerate_in_ovariectomized_women.png/427px-Estradiol_levels_after_an_intramuscular_injection_of_Climacteron_or_estradiol_valerate_in_ovariectomized_women.png)