Conjugated estrogens
| Drug class | Estrogen | |
|---|---|---|
| ATC code | ||
| Legal status | ||
| Legal status | ||
ECHA InfoCard | 100.031.987 | |
| (verify) | ||
Conjugated estrogens (CEs), or conjugated equine estrogens (CEEs), sold under the brand name Premarin among others, is an
Premarin, the major brand of CEEs in use, is manufactured by
Medical uses
CEEs are a form of
CEEs are specifically approved in countries such as the
: 60| Route/form | Estrogen | Low | Standard | High | |||
|---|---|---|---|---|---|---|---|
| Oral | Estradiol | 0.5–1 mg/day | 1–2 mg/day | 2–4 mg/day | |||
| Estradiol valerate | 0.5–1 mg/day | 1–2 mg/day | 2–4 mg/day | ||||
| Estradiol acetate | 0.45–0.9 mg/day | 0.9–1.8 mg/day | 1.8–3.6 mg/day | ||||
| Conjugated estrogens | 0.3–0.45 mg/day | 0.625 mg/day | 0.9–1.25 mg/day | ||||
| Esterified estrogens | 0.3–0.45 mg/day | 0.625 mg/day | 0.9–1.25 mg/day | ||||
| Estropipate | 0.75 mg/day | 1.5 mg/day | 3 mg/day | ||||
| Estriol | 1–2 mg/day | 2–4 mg/day | 4–8 mg/day | ||||
| Ethinylestradiola | 2.5–10 μg/day | 5–20 μg/day | – | ||||
| Nasal spray | Estradiol | 150 μg/day | 300 μg/day | 600 μg/day | |||
| Transdermal patch | Estradiol | 25 μg/dayb | 50 μg/dayb | 100 μg/dayb | |||
Transdermal gel |
Estradiol | 0.5 mg/day | 1–1.5 mg/day | 2–3 mg/day | |||
Vaginal |
Estradiol | 25 μg/day | – | – | |||
| Estriol | 30 μg/day | 0.5 mg 2x/week | 0.5 mg/day | ||||
SC injection |
Estradiol valerate | – | – | 4 mg 1x/4 weeks | |||
| Estradiol cypionate | 1 mg 1x/3–4 weeks | 3 mg 1x/3–4 weeks | 5 mg 1x/3–4 weeks | ||||
| Estradiol benzoate | 0.5 mg 1x/week | 1 mg 1x/week | 1.5 mg 1x/week | ||||
| SC implant | Estradiol | 25 mg 1x/6 months | 50 mg 1x/6 months | 100 mg 1x/6 months | |||
| Footnotes: a = No longer used or recommended, due to health concerns. b = As a single patch applied once or twice per week (worn for 3–4 days or 7 days), depending on the formulation. Note: Dosages are not necessarily equivalent. Sources: See template. | |||||||
Available forms
Natural CEEs, as Premarin, are available in the form of
Contraindications
Side effects
The most common
| Clinical outcome | Hypothesized effect on risk |
Estrogen and progestogen (CEs 0.625 mg/day p.o. + MPA 2.5 mg/day p.o.) (n = 16,608, with uterus, 5.2–5.6 years follow up) |
Estrogen alone (CEs 0.625 mg/day p.o.) (n = 10,739, no uterus, 6.8–7.1 years follow up) | ||||
|---|---|---|---|---|---|---|---|
| HR | 95% CI | AR
|
HR | 95% CI | AR
| ||
Coronary heart disease
|
Decreased | 1.24 | 1.00–1.54 | +6 / 10,000 PYs | 0.95 | 0.79–1.15 | −3 / 10,000 PYs |
| Stroke | Decreased | 1.31 | 1.02–1.68 | +8 / 10,000 PYs | 1.37 | 1.09–1.73 | +12 / 10,000 PYs |
| Pulmonary embolism | Increased | 2.13 | 1.45–3.11 | +10 / 10,000 PYs | 1.37 | 0.90–2.07 | +4 / 10,000 PYs |
Venous thromboembolism
|
Increased | 2.06 | 1.57–2.70 | +18 / 10,000 PYs | 1.32 | 0.99–1.75 | +8 / 10,000 PYs |
| Breast cancer | Increased | 1.24 | 1.02–1.50 | +8 / 10,000 PYs | 0.80 | 0.62–1.04 | −6 / 10,000 PYs |
| Colorectal cancer | Decreased | 0.56 | 0.38–0.81 | −7 / 10,000 PYs | 1.08 | 0.75–1.55 | +1 / 10,000 PYs |
| Endometrial cancer | – | 0.81 | 0.48–1.36 | −1 / 10,000 PYs | – | – | – |
| Hip fractures | Decreased | 0.67 | 0.47–0.96 | −5 / 10,000 PYs | 0.65 | 0.45–0.94 | −7 / 10,000 PYs |
| Total fractures | Decreased | 0.76 | 0.69–0.83 | −47 / 10,000 PYs | 0.71 | 0.64–0.80 | −53 / 10,000 PYs |
| Total mortality | Decreased | 0.98 | 0.82–1.18 | −1 / 10,000 PYs | 1.04 | 0.91–1.12 | +3 / 10,000 PYs |
| Global index | – | 1.15 | 1.03–1.28 | +19 / 10,000 PYs | 1.01 | 1.09–1.12 | +2 / 10,000 PYs |
| Diabetes | – | 0.79 | 0.67–0.93 | 0.88 | 0.77–1.01 | ||
| Gallbladder disease | Increased | 1.59 | 1.28–1.97 | 1.67 | 1.35–2.06 | ||
| Stress incontinence | – | 1.87 | 1.61–2.18 | 2.15 | 1.77–2.82 | ||
Urge incontinence
|
– | 1.15 | 0.99–1.34 | 1.32 | 1.10–1.58 | ||
| Peripheral artery disease | – | 0.89 | 0.63–1.25 | 1.32 | 0.99–1.77 | ||
| Probable dementia | Decreased | 2.05 | 1.21–3.48 | 1.49 | 0.83–2.66 | ||
| Abbreviations: CEs = conjugated estrogens. MPA = coronary heart disease, stroke, pulmonary embolism, breast cancer, colorectal cancer, endometrial cancer (estrogen plus progestogen group only), hip fractures, and death from other causes. Sources: See template.
| |||||||
| Type | Route | Medications | Odds ratio (95% CI) |
|---|---|---|---|
Menopausal hormone therapy |
Oral | Estradiol alone ≤1 mg/day >1 mg/day |
1.27 (1.16–1.39)* 1.22 (1.09–1.37)* 1.35 (1.18–1.55)* |
| Conjugated estrogens alone ≤0.625 mg/day >0.625 mg/day |
1.49 (1.39–1.60)* 1.40 (1.28–1.53)* 1.71 (1.51–1.93)* | ||
| Estradiol/medroxyprogesterone acetate | 1.44 (1.09–1.89)* | ||
| Estradiol/dydrogesterone ≤1 mg/day E2 >1 mg/day E2 |
1.18 (0.98–1.42) 1.12 (0.90–1.40) 1.34 (0.94–1.90) | ||
| Estradiol/norethisterone ≤1 mg/day E2 >1 mg/day E2 |
1.68 (1.57–1.80)* 1.38 (1.23–1.56)* 1.84 (1.69–2.00)* | ||
Estradiol/norgestrel or estradiol/drospirenone |
1.42 (1.00–2.03) | ||
| Conjugated estrogens/medroxyprogesterone acetate | 2.10 (1.92–2.31)* | ||
| Conjugated estrogens/norgestrel ≤0.625 mg/day CEEs >0.625 mg/day CEEs |
1.73 (1.57–1.91)* 1.53 (1.36–1.72)* 2.38 (1.99–2.85)* | ||
| Tibolone alone | 1.02 (0.90–1.15) | ||
| Raloxifene alone | 1.49 (1.24–1.79)* | ||
Transdermal |
Estradiol alone ≤50 μg/day >50 μg/day |
0.96 (0.88–1.04) 0.94 (0.85–1.03) 1.05 (0.88–1.24) | |
| Estradiol/progestogen | 0.88 (0.73–1.01) | ||
Vaginal |
Estradiol alone | 0.84 (0.73–0.97) | |
| Conjugated estrogens alone | 1.04 (0.76–1.43) | ||
Combined birth control |
Oral | Ethinylestradiol/norethisterone | 2.56 (2.15–3.06)* |
| Ethinylestradiol/levonorgestrel | 2.38 (2.18–2.59)* | ||
Ethinylestradiol/norgestimate |
2.53 (2.17–2.96)* | ||
| Ethinylestradiol/desogestrel | 4.28 (3.66–5.01)* | ||
| Ethinylestradiol/gestodene | 3.64 (3.00–4.43)* | ||
| Ethinylestradiol/drospirenone | 4.12 (3.43–4.96)* | ||
| Ethinylestradiol/cyproterone acetate | 4.27 (3.57–5.11)* | ||
| Notes: (1) Bioidentical progesterone was not included, but is known to be associated with no additional risk relative to estrogen alone. Footnotes: * = Statistically significant (p < 0.01). Sources: See template.
| |||
Overdose
Estrogens, including CEEs, are relatively safe in acute
Interactions
Inhibitors and inducers of cytochrome P450 enzymes may interact with CEEs.[citation needed]
Pharmacology
Pharmacodynamics

CEEs are a combination of
CEEs consists of the
A dosage of 0.625 mg/day oral CEEs has been found to increase SHBG levels by 100%.[41][42] For comparison, 1 mg/day oral estradiol increased SHBG levels by 45%, while 50 µg/day transdermal estradiol increased SHBG levels by 12%.[41][42] Ethinylestradiol is more potent in its effects on liver protein synthesis than either CEEs or estradiol, with 10 µg/day oral ethinylestradiol having been found to be approximately equivalent to 1.25 mg/day CEEs.[41]
| Compound | Synonym | Proportion (%) | Relative potency in the vagina (%) |
Relative potency in the uterus (%) |
ERα (%) |
RBA for ERβ (%) |
ERα / ERβ RBA ratio |
|---|---|---|---|---|---|---|---|
| Conjugated estrogens | – | 100 | 38 | 100 | – | – | – |
| Estrone | – | 49.1–61.5 | 30 | 32 | 26 | 52 | 0.50 |
| Equilin | Δ7-Estrone | 22.4–30.5 | 42 | 80 | 13 | 49 | 0.26 |
| 17α-Dihydroequilin | Δ7-17α-Estradiol | 13.5–19.5 | 0.06 | 2.6 | 41 | 32 | 1.30 |
| 17α-Estradiol | – | 2.5–9.5 | 0.11 | 3.5 | 19 | 42 | 0.45 |
| Δ8-Estrone | – | 3.5–3.9 | ? | ? | 19 | 32 | 0.60 |
| Equilenin | Δ6,8-Estrone | 2.2–2.8 | 1.3 | 11.4 | 15 | 20–29 | 0.50–0.75 |
| 17β-Dihydroequilin | Δ7-17β-Estradiol | 0.5–4.0 | 83 | 200 | 113 | 108 | 1.05 |
| 17α-Dihydroequilenin | Δ6,8-17α-Estradiol | 1.2–1.6 | 0.018 | 1.3 | 20 | 49 | 0.40 |
| 17β-Estradiol | – | 0.56–0.9 | 100 | ? | 100 | 100 | 1.00 |
| 17β-Dihydroequilenin | Δ6,8-17β-Estradiol | 0.5–0.7 | 0.21 | 9.4 | 68 | 90 | 0.75 |
| Δ8-17β-Estradiol | – | Small amounts | ? | ? | 68 | 72 | 0.94 |
| Notes: All listed compounds are present in conjugated estrogen products specifically in the form of the sodium salts of the sulfate esters (i.e., as sodium estrone sulfate, sodium equilin sulfate, etc.). Sources: See template. | |||||||
| Compound | Dosage for specific uses (mg usually)[a] | ||||||
|---|---|---|---|---|---|---|---|
| ETD[b] | EPD[b] | MSD[b] | MSD[c] | OID[c] | TSD[c] | ||
| Estradiol (non-micronized) | 30 | ≥120–300 | 120 | 6 | - | - | |
| Estradiol (micronized) | 6–12 | 60–80 | 14–42 | 1–2 | >5 | >8 | |
| Estradiol valerate | 6–12 | 60–80 | 14–42 | 1–2 | - | >8 | |
| Estradiol benzoate | - | 60–140 | - | - | - | - | |
| Estriol | ≥20 | 120–150[d] | 28–126 | 1–6 | >5 | - | |
| Estriol succinate | - | 140–150[d] | 28–126 | 2–6 | - | - | |
| Estrone sulfate | 12 | 60 | 42 | 2 | - | - | |
| Conjugated estrogens | 5–12 | 60–80 | 8.4–25 | 0.625–1.25 | >3.75 | 7.5 | |
| Ethinylestradiol | 200 μg | 1–2 | 280 μg | 20–40 μg | 100 μg | 100 μg | |
| Mestranol | 300 μg | 1.5–3.0 | 300–600 μg | 25–30 μg | >80 μg | - | |
| Quinestrol | 300 μg | 2–4 | 500 μg | 25–50 μg | - | - | |
| Methylestradiol | - | 2 | - | - | - | - | |
| Diethylstilbestrol | 2.5 | 20–30 | 11 | 0.5–2.0 | >5 | 3 | |
| DES dipropionate | - | 15–30 | - | - | - | - | |
| Dienestrol | 5 | 30–40 | 42 | 0.5–4.0 | - | - | |
| Dienestrol diacetate | 3–5 | 30–60 | - | - | - | - | |
| Hexestrol | - | 70–110 | - | - | - | - | |
| Chlorotrianisene | - | >100 | - | - | >48 | - | |
| Methallenestril | - | 400 | - | - | - | - | |
| Estrogen | HF |
VE | UCa | FSH | LH | HDL-C | SHBG | CBG |
AGT |
Liver |
|---|---|---|---|---|---|---|---|---|---|---|
| Estradiol | 1.0 | 1.0 | 1.0 | 1.0 | 1.0 | 1.0 | 1.0 | 1.0 | 1.0 | 1.0 |
| Estrone | ? | ? | ? | 0.3 | 0.3 | ? | ? | ? | ? | ? |
| Estriol | 0.3 | 0.3 | 0.1 | 0.3 | 0.3 | 0.2 | ? | ? | ? | 0.67 |
| Estrone sulfate | ? | 0.9 | 0.9 | 0.8–0.9 | 0.9 | 0.5 | 0.9 | 0.5–0.7 | 1.4–1.5 | 0.56–1.7 |
| Conjugated estrogens | 1.2 | 1.5 | 2.0 | 1.1–1.3 | 1.0 | 1.5 | 3.0–3.2 | 1.3–1.5 | 5.0 | 1.3–4.5 |
Equilin sulfate |
? | ? | 1.0 | ? | ? | 6.0 | 7.5 | 6.0 | 7.5 | ? |
| Ethinylestradiol | 120 | 150 | 400 | 60–150 | 100 | 400 | 500–600 | 500–600 | 350 | 2.9–5.0 |
| Diethylstilbestrol | ? | ? | ? | 2.9–3.4 | ? | ? | 26–28 | 25–37 | 20 | 5.7–7.5 |
Sources and footnotes
Notes: Values are ratios, with estradiol as standard (i.e., 1.0). Abbreviations: HF = Clinical relief of liver proteins. Liver = Ratio of liver estrogenic effects to general/systemic estrogenic effects (hot flashes/gonadotropins ). Sources: See template. | ||||||||||
Antigonadotropic effects
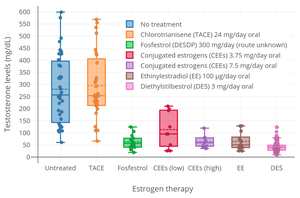
A preliminary study of ovulation inhibition in women found that oral CEEs was 33% effective at 1.25 mg/day and 94% at 3.75 mg/day.[63][64] A dosage of oral CEEs of 2.5 mg three times daily (7.5 mg/day total) has been found to suppress total testosterone levels in men to an equivalent extent as 3 mg/day oral diethylstilbestrol, which is the minimum dosage of diethylstilbestrol required to consistently suppress total testosterone levels into the castrate range (<50 ng/dL).[65]
Pharmacokinetics
CEEs are
Oral CEEs, at a daily dosage of 0.625 mg, achieve estrone and estradiol levels of 150 pg/mL and 30–50 pg/mL, respectively, while a daily oral dosage of 1.25 mg achieves levels of 120–200 pg/mL and 40–60 pg/mL of estrone and estradiol, respectively.
Eoncentrations of equilin that are very high relative to those of other estrogens are produced by typical clinical doses of CEEs.[72] With a dosage of 1.25 mg oral CEEs, equilin levels of 1,082 to 2,465 pg/mL have been observed.[72] The clinical significance of these levels of equilin is unknown.[72]
The active forms are
| Route | Dose | Time | E2 (↑Δ) | E1 (↑Δ) | Ratio | |
|---|---|---|---|---|---|---|
| Oral |
0.3 mg 0.625 mg 1.25 mg 1.25 mg 2.5 mg |
6 hours 6 hours 6 hours 1 hour 6 hours |
+20 pg/mL +50 pg/mL +70 pg/mL +35–58 pg/mL +160 pg/mL |
ND ND ND 110 pg/mL ND |
ND ND ND 0.32–0.52 ND | |
cream ) |
0.3 mg 0.625 mg 0.625 mg 1.25 mg 1.25 mg 2.5 mg |
ND ND ND 2 hours ND ND |
+4 pg/mL +13–29 pg/mL +17 pg/mL +25 pg/mL +27 pg/mL +32 pg/mL |
+20 pg/mL +29–55 pg/mL +45 pg/mL +50 pg/mL +110 pg/mL +40 pg/mL |
0.2 0.24–1.0 0.38 0.5 0.25 0.8 | |
Intravenous a |
20 mg | 5 min 30 min 60 min 120 min |
800 pg/mL 3000 pg/mL 3500 pg/mL 3100 pg/mL |
4500 pg/mL 24000 pg/mL 19000 pg/mL 10500 pg/mL |
1:5.3 1:8.1 1:5.5 1:3.4 | |
| Notes: a = Absolute levels, not change. Sources: See template. | ||||||
| Compound | RBA to (%)SHBG |
Bound to SHBG (%) |
Bound to albumin (%) |
Total bound (%) |
MCR (L/day/m2) |
|---|---|---|---|---|---|
| 17β-Estradiol | 50 | 37 | 61 | 98 | 580 |
| Estrone | 12 | 16 | 80 | 96 | 1050 |
| Estriol | 0.3 | 1 | 91 | 92 | 1110 |
| Estrone sulfate | 0 | 0 | 99 | 99 | 80 |
| 17β-Dihydroequilin | 30 | ? | ? | ? | 1250 |
| Equilin | 8 | 26 | 13 | ? | 2640 |
17β-Dihydroequilin sulfate |
0 | ? | ? | ? | 375 |
Equilin sulfate |
0 | ? | ? | ? | 175 |
| Δ8-Estrone | ? | ? | ? | ? | 1710 |
| Notes: RBA for SHBG (%) is compared to 100% for testosterone. Sources: See template. | |||||
Chemistry
CEEs are
History
Conjugated estriol, an extract of the urine of pregnant women and sold under the brand names Progynon and Emmenin in the 1930s, was the predecessor of Premarin.[74] Both of these products contained conjugated estrogens similarly to Premarin, but the estrogens were human estrogens as opposed to equine estrogens and the composition differed. The major active ingredient in Progynon and Emmenin was estriol glucuronide.
Estrone sulfate was first isolated from the urine of pregnant mares in the late 1930s by researchers in the Department of Biochemistry at
Conjugated estrogens was introduced for medical use under the brand name Premarin in Canada in 1941, in the United States in 1942, and in the United Kingdom in 1956.[80]
The manufacturer of Premarin secretly paid
Society and culture
Names
Estrogens, conjugated is the
CEEs are marketed under a large number of brand names throughout the world.[6] The major brand name of the natural form of CEEs manufactured from the urine of pregnant mares is Premarin.[6] Major brand names of fully synthetic versions of CEEs include Cenestin and Enjuvia in the United States and C.E.S. and Congest in Canada.[6][8][9] CEEs are also formulated in combination with progestins.[6] Major brand names of CEEs in combination with medroxyprogesterone acetate include Prempro and Premphase in the United States, Premplus in Canada, Premique in the United Kingdom and Ireland, Premia in Australia and New Zealand, and Premelle in South Africa.[6][86] Prempak-C is a combination of CEEs and norgestrel which is used in the United Kingdom and Ireland, and Prempak N is a combination of CEEs and medrogestone which is used in South Africa.[6] Many of the aforementioned brand names are also used in other, non-English-speaking countries.[6]
Availability
CEEs are marketed and available widely throughout the world.[6][26] This includes in all English-speaking countries, throughout the European Union, Latin America, Asia, and elsewhere in the world.[6][26]
Health effects
Research starting in 1975 showed substantially increased risk of
Litigation
This drug has been the subject of litigation; more than 13,000 people have sued Wyeth between 2002 and 2009. Wyeth and Pharmacia & Upjohn prevailed in the vast majority of hormone therapy cases previously set for trial through a combination of rulings by judges, verdicts by juries, and dismissals by plaintiffs themselves.[92] Of the company's losses, two of the jury verdicts were reversed post-trial and others are being challenged on appeal. Wyeth also won five summary judgments on Prempro cases and had 15 cases voluntarily dismissed by plaintiffs. The company won dismissals in another 3,000 cases.[93] In 2006, Mary Daniel, in a trial in Philadelphia, was awarded $1.5 million in compensatory damages as well as undisclosed punitive damages. As of 2010, Wyeth had won the last four of five cases, most recently in Virginia, finding that they were not responsible for the breast cancer of plaintiff Georgia Torkie-Tork.[94] Wyeth has been quoted as saying "many risk factors associated with breast cancer have been identified, but science cannot establish what role any particular risk factor or combination play in any individual woman's breast cancer."[95] Wyeth's counsel in the case also noted that in the WHI trial, 99.62% of women took the drug and "did not get breast cancer."[93]
Animal welfare
Notes
References
- ^ S2CID 24616324.
- ^ a b c d e "Drugs@FDA: FDA Approved Drug Products". United States Food and Drug Administration. Retrieved 19 February 2018.
- FDA. Retrieved 22 October 2023.
- ^ a b c d e f g h "PREMARIN- estrogens, conjugated tablet, film coated Wyeth Pharmaceuticals LLC, a subsidiary of Pfizer Inc". labeling.pfizer.com. Retrieved 3 June 2019.
- ^ a b c d "Conjugated estrogens".
- ^ ISBN 978-0-85369-840-1.
- ^ ISBN 978-1-4511-4847-3.
- ^ ISBN 978-0-323-08578-6.
- ^ ISBN 978-92-832-1291-1.
- S2CID 24155838.
- S2CID 5084606.
- ^ PMID 24176763.
- ^ ISBN 978-3-319-59317-3.
- S2CID 4771048.
- S2CID 30292136.
- S2CID 2060730.
- ^ "The Top 300 of 2020". ClinCalc. Retrieved 7 October 2022.
- ^ "Estrogens, Conjugated - Drug Usage Statistics". ClinCalc. Retrieved 7 October 2022.
- ISBN 978-0-340-80997-6.
- ISBN 978-1-139-47200-5. Retrieved 7 May 2015.
- PMID 24830593.
- PMID 24870721.
- ISBN 978-1-284-09137-3. Retrieved 7 May 2015.
- ISBN 978-3-642-60107-1.
- ISBN 978-1-85317-422-3.
- ^ a b c "Premarin".
- ISBN 978-94-009-8195-9.
- ^ Piersol GM (1975). The Cyclopedia of Medicine, Surgery, Specialties. F. A. Davis Company.
- ISBN 978-1-59259-715-4.
- ISBN 978-1-4377-1035-9.
- ^ "Premarin (Conjugated estrogens) Vaginal Cream". Food and Drug Administration. Archived from the original on 26 October 2016. Retrieved 20 February 2018.
- ^ PMID 26327865.
- PMID 24081194.
- PMID 15467060.
- PMID 27998619.
- S2CID 22306721.
- ISBN 978-1-4612-5525-3.
- ISBN 978-1-4614-6837-0.
- ISBN 978-0-387-94739-6.
Conjugated estrogens are absorbed with peak levels at 4 hours and a half-life of approximately 12 hours.
- PMID 17443713.
- ^ PMID 16915215.
- ^ S2CID 3076514.
- PMID 2215269.
- PMID 559617.
- ISBN 978-3-642-75101-1.
- ISBN 978-3-11-024568-4.
- ISBN 978-3-662-00942-0.
- ISBN 978-94-009-8195-9.
- ISBN 978-3-11-150424-7.
- PMID 779393.
- ISSN 0001-6349.
- ISSN 0172-777X.
- PMID 29603164.
- PMID 29756046.
- PMID 15432047.
- PMID 14902290.
- ISSN 0001-6349.
There is no doubt that the conversion of the endometrium with injections of both synthetic and native estrogenic hormone preparations succeeds, but the opinion whether native, orally administered preparations can produce a proliferation mucosa changes with different authors. PEDERSEN-BJERGAARD (1939) was able to show that 90% of the folliculin taken up in the blood of the vena portae is inactivated in the liver. Neither KAUFMANN (1933, 1935), RAUSCHER (1939, 1942) nor HERRNBERGER (1941) succeeded in bringing a castration endometrium into proliferation using large doses of orally administered preparations of estrone or estradiol. Other results are reported by NEUSTAEDTER (1939), LAUTERWEIN (1940) and FERIN (1941); they succeeded in converting an atrophic castration endometrium into an unambiguous proliferation mucosa with 120–300 oestradiol or with 380 oestrone.
- ISBN 978-3-642-57636-2.
- ^ Martinez-Manautou J, Rudel HW (1966). "Antiovulatory Activity of Several Synthetic and Natural Estrogens". In Robert Benjamin Greenblatt (ed.). Ovulation: Stimulation, Suppression, and Detection. Lippincott. pp. 243–253.
- ISBN 978-3-642-49506-9.
- PMID 13370006.
- ^ PMID 4359746.
- ISBN 978-0-397-59010-0.
- ISBN 978-3-642-49506-9.)
{{cite book}}: CS1 maint: DOI inactive as of February 2024 (link - S2CID 4492779.
- ^ PMID 8842581.
- ^ ISBN 978-0-08-055309-2.
- ISBN 978-94-009-4145-8.
- ISBN 978-0-8422-7205-6.
- PMID 7231202.
- PMID 3653846.
- ^ PMID 6277697.
- PMID 18599548.
- ISBN 978-0-7735-2501-6.
- .
- ^ a b c Kling J (October 2000). "The Strange Case of Premarin". Modern Drug Discovery. 3 (8): 46–52.
- ^ "Federal register [microform]". Washington : [Office of the Federal Register, National Archives and Records Service, General Services Administration : Distributed by the Supt. of Docs., U.S. G.P.O.] 3 June 1972 – via Internet Archive.
- ^ National Institutes of Health Consensus Development Conference Statement. 2–4 April 1984 Osteoporosis Archived 21 November 2005 at the Wayback Machine
- ^ Food and Drug Administration. 5 May 1997 Conjugated Estrogens - Letter from Dr. Janet Woodcock: Approvability of a Synthetic Generic Version of Premarin
- ISBN 978-1-107-45182-7.
Premarin (Pregnant Mares Urine) was introduced in Canada in 1941, in the USA in 1942 and in the UK in 1956.
- ISSN 0362-4331. Retrieved 7 February 2023.
Every woman has the right — indeed the duty — to counteract the chemical castration that befalls her during her middle years," the gynecologist Robert Wilson wrote in 1966. The U.S. Food and Drug Administration approved the first hormone-therapy drug in 1942, but Wilson's blockbuster book, "Feminine Forever," can be considered a kind of historical landmark...Within a decade of the book's publication, Premarin — a mix of estrogens derived from the urine of pregnant horses — was the fifth-most-prescribed drug in the United States. (Decades later, it was revealed that Wilson received funding from the pharmaceutical company that sold Premarin.)
- ^ "ChemIDplus - 12126-59-9 - QTTMOCOWZLSYSV-QWAPEVOJSA-M - Estrogens, conjugated [USP:JAN] - Similar structures search, synonyms, formulas, resource links, and other chemical information".
- PMID 23217066.
- PMID 15845914.
- PMID 21189221.
- ISBN 978-1-4027-4446-4.
- PMID 171569.
- PMID 190887.
- ^ Singer N, Wilson D (12 December 2009). "Menopause, as Brought to You by Big Pharma". New York Times.
- PMID 16186467.
- ^ "Earnings Results for the 2006 Fourth Quarter and Full Year" (PDF) (Press release). Wyeth. Archived from the original (PDF) on 27 November 2007. Retrieved 20 February 2018.
- ^ "Pfizer Statement on Prempro". Indy News Channel. 24 November 2009. Archived from the original on 23 February 2012.
- ^ a b Feeley J (24 February 2010). "Pfizer wins trial over claim Prempro caused cancer". Bloomberg.
- ^ "Pfizer properly warned about Prempro risks, jury finds". 3 December 2010.
- ^ "Legal Intelligencer: Philadelphia jury returns defense verdict in HRT case, Amaris Elliott Engel".
- ^ Morrison K (19 January 2004). "The HRT horses". msnbc.com.
Further reading
- Bhavnani BR (November 1988). "The saga of the ring B unsaturated equine estrogens". Endocrine Reviews. 9 (4): 396–416. PMID 3065072.
- Ansbacher R (April 1993). "Bioequivalence of conjugated estrogen products". Clinical Pharmacokinetics. 24 (4): 271–274. S2CID 7681617.
- O'Connell MB (September 1995). "Pharmacokinetic and pharmacologic variation between different estrogen products". Journal of Clinical Pharmacology. 35 (9S): 18S–24S. S2CID 10159196.
- Egarter C, Geurts P, Boschitsch E, Speiser P, Huber J (April 1996). "The effects of estradiol valerate plus medroxyprogesterone acetate and conjugated estrogens plus medrogestone on climacteric symptoms and metabolic variables in perimenopausal women". Acta Obstetricia et Gynecologica Scandinavica. 75 (4): 386–393. S2CID 44498140.
- Bhavnani BR (January 1998). "Pharmacokinetics and pharmacodynamics of conjugated equine estrogens: chemistry and metabolism". Proceedings of the Society for Experimental Biology and Medicine. 217 (1): 6–16. S2CID 45177839.
- Gruber DM, Huber JC (December 1999). "Conjugated estrogens--the natural SERMs". Gynecological Endocrinology. 13 (Suppl 6): 9–12. PMID 10862263.
- Campagnoli C, Ambroggio S, Biglia N, Sismondi P (December 1999). "Conjugated estrogens and breast cancer risk". Gynecological Endocrinology. 13 (Suppl 6): 13–19. PMID 10862264.
- Bhavnani BR (June 2003). "Estrogens and menopause: pharmacology of conjugated equine estrogens and their potential role in the prevention of neurodegenerative diseases such as Alzheimer's". The Journal of Steroid Biochemistry and Molecular Biology. 85 (2–5): 473–482. S2CID 45552896.
- Ortmann J, Traupe T, Vetter W, Barton M (May 2004). "[Postmenopausal hormone replacement therapy and cardiovascular risk: role of conjugated equine estrogens and medroxyprogesterone acetate]". Praxis (in German). 93 (21): 904–914. PMID 15216975.
- Kuhl H (August 2005). "Pharmacology of estrogens and progestogens: influence of different routes of administration". Climacteric. 8 (Suppl 1): 3–63. S2CID 24616324.
- Kurabayashi T (November 2007). "[New evidence of conjugated estrogen and 17beta-estradiol for treatment and prevention of osteoporosis]". Nihon Rinsho. Japanese Journal of Clinical Medicine (in Japanese). 65 (Suppl 9): 369–373. PMID 18161134.
- Lamba G, Kaur H, Adapa S, Shah D, Malhotra BK, Rafiyath SM, et al. (June 2013). "Use of conjugated estrogens in life-threatening gastrointestinal bleeding in hemodialysis patients--a review". Clinical and Applied Thrombosis/Hemostasis. 19 (3): 334–337. S2CID 30468265.
- Mirkin S, Komm BS, Pickar JH (January 2014). "Conjugated estrogens for the treatment of menopausal symptoms: a review of safety data". Expert Opinion on Drug Safety. 13 (1): 45–56. S2CID 24379298.
- Bhavnani BR, Stanczyk FZ (July 2014). "Pharmacology of conjugated equine estrogens: efficacy, safety and mechanism of action". The Journal of Steroid Biochemistry and Molecular Biology. 142: 16–29. S2CID 1360563.
- Mattison DR, Karyakina N, Goodman M, LaKind JS (September 2014). "Pharmaco- and toxicokinetics of selected exogenous and endogenous estrogens: a review of the data and identification of knowledge gaps". Critical Reviews in Toxicology. 44 (8): 696–724. S2CID 11212469.
External links
- WHI Follow-up Study Confirms Health Risks of Long-Term Combination Hormone Therapy Outweigh Benefits for Postmenopausal Women NIH press release, 4 March 2008

