Estetrol (medication)
 | |
 | |
| Clinical data | |
|---|---|
| Trade names | With drospirenone: Estelle, Nextstellis |
| Other names | Oestetrol; E4; 15α-Hydroxyestriol; Estra-1,3,5(10)-triene-3,15α,16α,17β-tetrol |
| Pregnancy category |
|
| Identifiers | |
| |
JSmol) | |
| Solubility in water | 1.38 |
| |
| |
| (verify) | |
Estetrol (E4) is an
Estetrol is a
Estetrol was first discovered in 1965, and basic research continued up until 1984.[2][13] It started to be studied again as well as investigated for potential medical use in 2001, and by 2008, was of major interest for possible medical use.[2][3] As of 2021, estetrol is in mid- to late-stage clinical development for a variety of indications.[7][8]
| Route/form | Estrogen | Low | Standard | High | |||
|---|---|---|---|---|---|---|---|
| Oral | Estradiol | 0.5–1 mg/day | 1–2 mg/day | 2–4 mg/day | |||
| Estradiol valerate | 0.5–1 mg/day | 1–2 mg/day | 2–4 mg/day | ||||
| Estradiol acetate | 0.45–0.9 mg/day | 0.9–1.8 mg/day | 1.8–3.6 mg/day | ||||
| Conjugated estrogens | 0.3–0.45 mg/day | 0.625 mg/day | 0.9–1.25 mg/day | ||||
| Esterified estrogens | 0.3–0.45 mg/day | 0.625 mg/day | 0.9–1.25 mg/day | ||||
| Estropipate | 0.75 mg/day | 1.5 mg/day | 3 mg/day | ||||
| Estriol | 1–2 mg/day | 2–4 mg/day | 4–8 mg/day | ||||
| Ethinylestradiola | 2.5–10 μg/day | 5–20 μg/day | – | ||||
| Nasal spray | Estradiol | 150 μg/day | 300 μg/day | 600 μg/day | |||
| Transdermal patch | Estradiol | 25 μg/dayb | 50 μg/dayb | 100 μg/dayb | |||
Transdermal gel |
Estradiol | 0.5 mg/day | 1–1.5 mg/day | 2–3 mg/day | |||
Vaginal |
Estradiol | 25 μg/day | – | – | |||
| Estriol | 30 μg/day | 0.5 mg 2x/week | 0.5 mg/day | ||||
SC injection |
Estradiol valerate | – | – | 4 mg 1x/4 weeks | |||
| Estradiol cypionate | 1 mg 1x/3–4 weeks | 3 mg 1x/3–4 weeks | 5 mg 1x/3–4 weeks | ||||
| Estradiol benzoate | 0.5 mg 1x/week | 1 mg 1x/week | 1.5 mg 1x/week | ||||
| SC implant | Estradiol | 25 mg 1x/6 months | 50 mg 1x/6 months | 100 mg 1x/6 months | |||
| Footnotes: a = No longer used or recommended, due to health concerns. b = As a single patch applied once or twice per week (worn for 3–4 days or 7 days), depending on the formulation. Note: Dosages are not necessarily equivalent. Sources: See template. | |||||||
Available forms
Estetrol is available in combination with drospirenone in the following formulations, brand names and indications:
- Estetrol (as monohydrate) 15 mg and drospirenone 3 mg Nextstellis (CA, US and Australia) – combined oral contraception
- Estetrol (as monohydrate) 15 mg and drospirenone 3 mg Drovelis (EU) – combined oral contraception
- Estetrol (as monohydrate) 15 mg and drospirenone 3 mg Lydisilka (EU) – combined oral contraception
Side effects
Minimal
Estetrol-containing
Pharmacology
Pharmacodynamics
Estetrol is an
Estetrol shows high
| Ligand | Other names | Relative binding affinities (RBA, %)a |
Absolute binding affinities (Ki, nM)a |
Action | ||
|---|---|---|---|---|---|---|
ERα |
ERβ |
ERα |
ERβ
| |||
| Estradiol | E2; 17β-Estradiol | 100 | 100 | 0.115 (0.04–0.24) | 0.15 (0.10–2.08) | Estrogen |
| Estrone | E1; 17-Ketoestradiol | 16.39 (0.7–60) | 6.5 (1.36–52) | 0.445 (0.3–1.01) | 1.75 (0.35–9.24) | Estrogen |
| Estriol | E3; 16α-OH-17β-E2 | 12.65 (4.03–56) | 26 (14.0–44.6) | 0.45 (0.35–1.4) | 0.7 (0.63–0.7) | Estrogen |
| Estetrol | E4; 15α,16α-Di-OH-17β-E2 | 4.0 | 3.0 | 4.9 | 19 | Estrogen |
| Alfatradiol | 17α-Estradiol | 20.5 (7–80.1) | 8.195 (2–42) | 0.2–0.52 | 0.43–1.2 | Metabolite |
16-Epiestriol |
16β-Hydroxy-17β-estradiol | 7.795 (4.94–63) | 50 | ? | ? | Metabolite |
17-Epiestriol |
16α-Hydroxy-17α-estradiol | 55.45 (29–103) | 79–80 | ? | ? | Metabolite |
16,17-Epiestriol |
16β-Hydroxy-17α-estradiol | 1.0 | 13 | ? | ? | Metabolite |
| 2-Hydroxyestradiol | 2-OH-E2 | 22 (7–81) | 11–35 | 2.5 | 1.3 | Metabolite |
| 2-Methoxyestradiol | 2-MeO-E2 | 0.0027–2.0 | 1.0 | ? | ? | Metabolite |
| 4-Hydroxyestradiol | 4-OH-E2 | 13 (8–70) | 7–56 | 1.0 | 1.9 | Metabolite |
| 4-Methoxyestradiol | 4-MeO-E2 | 2.0 | 1.0 | ? | ? | Metabolite |
| 2-Hydroxyestrone | 2-OH-E1 | 2.0–4.0 | 0.2–0.4 | ? | ? | Metabolite |
| 2-Methoxyestrone | 2-MeO-E1 | <0.001–<1 | <1 | ? | ? | Metabolite |
| 4-Hydroxyestrone | 4-OH-E1 | 1.0–2.0 | 1.0 | ? | ? | Metabolite |
| 4-Methoxyestrone | 4-MeO-E1 | <1 | <1 | ? | ? | Metabolite |
| 16α-Hydroxyestrone | 16α-OH-E1; 17-Ketoestriol | 2.0–6.5 | 35 | ? | ? | Metabolite |
| 2-Hydroxyestriol | 2-OH-E3 | 2.0 | 1.0 | ? | ? | Metabolite |
| 4-Methoxyestriol | 4-MeO-E3 | 1.0 | 1.0 | ? | ? | Metabolite |
| Estradiol sulfate | E2S; Estradiol 3-sulfate | <1 | <1 | ? | ? | Metabolite |
| Estradiol disulfate | Estradiol 3,17β-disulfate | 0.0004 | ? | ? | ? | Metabolite |
| Estradiol 3-glucuronide | E2-3G | 0.0079 | ? | ? | ? | Metabolite |
Estradiol 17β-glucuronide |
E2-17G | 0.0015 | ? | ? | ? | Metabolite |
| Estradiol 3-gluc. 17β-sulfate | E2-3G-17S | 0.0001 | ? | ? | ? | Metabolite |
| Estrone sulfate | E1S; Estrone 3-sulfate | <1 | <1 | >10 | >10 | Metabolite |
| Estradiol benzoate | EB; Estradiol 3-benzoate | 10 | ? | ? | ? | Estrogen |
| Estradiol 17β-benzoate | E2-17B | 11.3 | 32.6 | ? | ? | Estrogen |
| Estrone methyl ether | Estrone 3-methyl ether | 0.145 | ? | ? | ? | Estrogen |
| ent-Estradiol | 1-Estradiol | 1.31–12.34 | 9.44–80.07 | ? | ? | Estrogen |
| Equilin | 7-Dehydroestrone | 13 (4.0–28.9) | 13.0–49 | 0.79 | 0.36 | Estrogen |
| Equilenin | 6,8-Didehydroestrone | 2.0–15 | 7.0–20 | 0.64 | 0.62 | Estrogen |
| 17β-Dihydroequilin | 7-Dehydro-17β-estradiol | 7.9–113 | 7.9–108 | 0.09 | 0.17 | Estrogen |
| 17α-Dihydroequilin | 7-Dehydro-17α-estradiol | 18.6 (18–41) | 14–32 | 0.24 | 0.57 | Estrogen |
| 17β-Dihydroequilenin | 6,8-Didehydro-17β-estradiol | 35–68 | 90–100 | 0.15 | 0.20 | Estrogen |
| 17α-Dihydroequilenin | 6,8-Didehydro-17α-estradiol | 20 | 49 | 0.50 | 0.37 | Estrogen |
| Δ8-Estradiol | 8,9-Dehydro-17β-estradiol | 68 | 72 | 0.15 | 0.25 | Estrogen |
| Δ8-Estrone | 8,9-Dehydroestrone | 19 | 32 | 0.52 | 0.57 | Estrogen |
| Ethinylestradiol | EE; 17α-Ethynyl-17β-E2 | 120.9 (68.8–480) | 44.4 (2.0–144) | 0.02–0.05 | 0.29–0.81 | Estrogen |
| Mestranol | EE 3-methyl ether | ? | 2.5 | ? | ? | Estrogen |
| Moxestrol | RU-2858; 11β-Methoxy-EE | 35–43 | 5–20 | 0.5 | 2.6 | Estrogen |
| Methylestradiol | 17α-Methyl-17β-estradiol | 70 | 44 | ? | ? | Estrogen |
| Diethylstilbestrol | DES; Stilbestrol | 129.5 (89.1–468) | 219.63 (61.2–295) | 0.04 | 0.05 | Estrogen |
| Hexestrol | Dihydrodiethylstilbestrol | 153.6 (31–302) | 60–234 | 0.06 | 0.06 | Estrogen |
| Dienestrol | Dehydrostilbestrol | 37 (20.4–223) | 56–404 | 0.05 | 0.03 | Estrogen |
| Benzestrol (B2) | – | 114 | ? | ? | ? | Estrogen |
| Chlorotrianisene | TACE | 1.74 | ? | 15.30 | ? | Estrogen |
| Triphenylethylene | TPE | 0.074 | ? | ? | ? | Estrogen |
| Triphenylbromoethylene | TPBE | 2.69 | ? | ? | ? | Estrogen |
| Tamoxifen | ICI-46,474 | 3 (0.1–47) | 3.33 (0.28–6) | 3.4–9.69 | 2.5 | SERM |
| Afimoxifene | 4-Hydroxytamoxifen; 4-OHT | 100.1 (1.7–257) | 10 (0.98–339) | 2.3 (0.1–3.61) | 0.04–4.8 | SERM |
| Toremifene | 4-Chlorotamoxifen; 4-CT | ? | ? | 7.14–20.3 | 15.4 | SERM |
| Clomifene | MRL-41 | 25 (19.2–37.2) | 12 | 0.9 | 1.2 | SERM |
| Cyclofenil | F-6066; Sexovid | 151–152 | 243 | ? | ? | SERM |
| Nafoxidine | U-11,000A | 30.9–44 | 16 | 0.3 | 0.8 | SERM |
| Raloxifene | – | 41.2 (7.8–69) | 5.34 (0.54–16) | 0.188–0.52 | 20.2 | SERM |
| Arzoxifene | LY-353,381 | ? | ? | 0.179 | ? | SERM |
| Lasofoxifene | CP-336,156 | 10.2–166 | 19.0 | 0.229 | ? | SERM |
| Ormeloxifene | Centchroman | ? | ? | 0.313 | ? | SERM |
| Levormeloxifene | 6720-CDRI; NNC-460,020 | 1.55 | 1.88 | ? | ? | SERM |
| Ospemifene | Deaminohydroxytoremifene | 0.82–2.63 | 0.59–1.22 | ? | ? | SERM |
| Bazedoxifene | – | ? | ? | 0.053 | ? | SERM |
| Etacstil | GW-5638 | 4.30 | 11.5 | ? | ? | SERM |
ICI-164,384 |
– | 63.5 (3.70–97.7) | 166 | 0.2 | 0.08 | Antiestrogen |
| Fulvestrant | ICI-182,780 | 43.5 (9.4–325) | 21.65 (2.05–40.5) | 0.42 | 1.3 | Antiestrogen |
| Propylpyrazoletriol | PPT | 49 (10.0–89.1) | 0.12 | 0.40 | 92.8 | ERα agonist |
| 16α-LE2 | 16α-Lactone-17β-estradiol | 14.6–57 | 0.089 | 0.27 | 131 | ERα agonist |
| 16α-Iodo-E2 | 16α-Iodo-17β-estradiol | 30.2 | 2.30 | ? | ? | ERα agonist |
| Methylpiperidinopyrazole | MPP | 11 | 0.05 | ? | ? | ERα antagonist |
| Diarylpropionitrile | DPN | 0.12–0.25 | 6.6–18 | 32.4 | 1.7 | ERβ agonist |
| 8β-VE2 | 8β-Vinyl-17β-estradiol | 0.35 | 22.0–83 | 12.9 | 0.50 | ERβ agonist |
| Prinaberel | ERB-041; WAY-202,041 | 0.27 | 67–72 | ? | ? | ERβ agonist |
| ERB-196 | WAY-202,196 | ? | 180 | ? | ? | ERβ agonist |
| Erteberel | SERBA-1; LY-500,307 | ? | ? | 2.68 | 0.19 | ERβ agonist |
| SERBA-2 | – | ? | ? | 14.5 | 1.54 | ERβ agonist |
| Coumestrol | – | 9.225 (0.0117–94) | 64.125 (0.41–185) | 0.14–80.0 | 0.07–27.0 | Xenoestrogen |
| Genistein | – | 0.445 (0.0012–16) | 33.42 (0.86–87) | 2.6–126 | 0.3–12.8 | Xenoestrogen |
| Equol | – | 0.2–0.287 | 0.85 (0.10–2.85) | ? | ? | Xenoestrogen |
| Daidzein | – | 0.07 (0.0018–9.3) | 0.7865 (0.04–17.1) | 2.0 | 85.3 | Xenoestrogen |
| Biochanin A | – | 0.04 (0.022–0.15) | 0.6225 (0.010–1.2) | 174 | 8.9 | Xenoestrogen |
| Kaempferol | – | 0.07 (0.029–0.10) | 2.2 (0.002–3.00) | ? | ? | Xenoestrogen |
| Naringenin | – | 0.0054 (<0.001–0.01) | 0.15 (0.11–0.33) | ? | ? | Xenoestrogen |
| 8-Prenylnaringenin | 8-PN | 4.4 | ? | ? | ? | Xenoestrogen |
| Quercetin | – | <0.001–0.01 | 0.002–0.040 | ? | ? | Xenoestrogen |
| Ipriflavone | – | <0.01 | <0.01 | ? | ? | Xenoestrogen |
| Miroestrol | – | 0.39 | ? | ? | ? | Xenoestrogen |
Deoxymiroestrol |
– | 2.0 | ? | ? | ? | Xenoestrogen |
β-Sitosterol |
– | <0.001–0.0875 | <0.001–0.016 | ? | ? | Xenoestrogen |
| Resveratrol | – | <0.001–0.0032 | ? | ? | ? | Xenoestrogen |
| α-Zearalenol | – | 48 (13–52.5) | ? | ? | ? | Xenoestrogen |
| β-Zearalenol | – | 0.6 (0.032–13) | ? | ? | ? | Xenoestrogen |
| Zeranol | α-Zearalanol | 48–111 | ? | ? | ? | Xenoestrogen |
| Taleranol | β-Zearalanol | 16 (13–17.8) | 14 | 0.8 | 0.9 | Xenoestrogen |
| Zearalenone | ZEN | 7.68 (2.04–28) | 9.45 (2.43–31.5) | ? | ? | Xenoestrogen |
| Zearalanone | ZAN | 0.51 | ? | ? | ? | Xenoestrogen |
| Bisphenol A | BPA | 0.0315 (0.008–1.0) | 0.135 (0.002–4.23) | 195 | 35 | Xenoestrogen |
| Endosulfan | EDS | <0.001–<0.01 | <0.01 | ? | ? | Xenoestrogen |
Kepone |
Chlordecone | 0.0069–0.2 | ? | ? | ? | Xenoestrogen |
o,p'-DDT |
– | 0.0073–0.4 | ? | ? | ? | Xenoestrogen |
p,p'-DDT |
– | 0.03 | ? | ? | ? | Xenoestrogen |
| Methoxychlor | p,p'-Dimethoxy-DDT | 0.01 (<0.001–0.02) | 0.01–0.13 | ? | ? | Xenoestrogen |
| HPTE | Hydroxychlor; p,p'-OH-DDT | 1.2–1.7 | ? | ? | ? | Xenoestrogen |
| Testosterone | T; 4-Androstenolone | <0.0001–<0.01 | <0.002–0.040 | >5000 | >5000 | Androgen |
| Dihydrotestosterone | DHT; 5α-Androstanolone | 0.01 (<0.001–0.05) | 0.0059–0.17 | 221–>5000 | 73–1688 | Androgen |
| Nandrolone | 19-Nortestosterone; 19-NT | 0.01 | 0.23 | 765 | 53 | Androgen |
| Dehydroepiandrosterone | DHEA; Prasterone | 0.038 (<0.001–0.04) | 0.019–0.07 | 245–1053 | 163–515 | Androgen |
5-Androstenediol |
A5; Androstenediol | 6 | 17 | 3.6 | 0.9 | Androgen |
| 4-Androstenediol | – | 0.5 | 0.6 | 23 | 19 | Androgen |
4-Androstenedione |
A4; Androstenedione | <0.01 | <0.01 | >10000 | >10000 | Androgen |
| 3α-Androstanediol | 3α-Adiol | 0.07 | 0.3 | 260 | 48 | Androgen |
| 3β-Androstanediol | 3β-Adiol | 3 | 7 | 6 | 2 | Androgen |
| Androstanedione | 5α-Androstanedione | <0.01 | <0.01 | >10000 | >10000 | Androgen |
| Etiocholanedione | 5β-Androstanedione | <0.01 | <0.01 | >10000 | >10000 | Androgen |
| Methyltestosterone | 17α-Methyltestosterone | <0.0001 | ? | ? | ? | Androgen |
Ethinyl-3α-androstanediol |
17α-Ethynyl-3α-adiol | 4.0 | <0.07 | ? | ? | Estrogen |
Ethinyl-3β-androstanediol |
17α-Ethynyl-3β-adiol | 50 | 5.6 | ? | ? | Estrogen |
| Progesterone | P4; 4-Pregnenedione | <0.001–0.6 | <0.001–0.010 | ? | ? | Progestogen |
| Norethisterone | NET; 17α-Ethynyl-19-NT | 0.085 (0.0015–<0.1) | 0.1 (0.01–0.3) | 152 | 1084 | Progestogen |
Norethynodrel |
5(10)-Norethisterone | 0.5 (0.3–0.7) | <0.1–0.22 | 14 | 53 | Progestogen |
| Tibolone | 7α-Methylnorethynodrel | 0.5 (0.45–2.0) | 0.2–0.076 | ? | ? | Progestogen |
| Δ4-Tibolone | 7α-Methylnorethisterone | 0.069–<0.1 | 0.027–<0.1 | ? | ? | Progestogen |
| 3α-Hydroxytibolone | – | 2.5 (1.06–5.0) | 0.6–0.8 | ? | ? | Progestogen |
| 3β-Hydroxytibolone | – | 1.6 (0.75–1.9) | 0.070–0.1 | ? | ? | Progestogen |
| Footnotes: a = (1) ERβ proteins (except the ERβ values from Kuiper et al. (1997), which are rat ERβ). Sources: See template page.
| ||||||
| Estrogen | Relative binding affinities (%)
| ||||||
|---|---|---|---|---|---|---|---|
| ER | AR | PR | GR | MR | SHBG | CBG
| |
| Estradiol | 100 | 7.9 | 2.6 | 0.6 | 0.13 | 8.7–12 | <0.1 |
| Estradiol benzoate | ? | ? | ? | ? | ? | <0.1–0.16 | <0.1 |
| Estradiol valerate | 2 | ? | ? | ? | ? | ? | ? |
| Estrone | 11–35 | <1 | <1 | <1 | <1 | 2.7 | <0.1 |
| Estrone sulfate | 2 | 2 | ? | ? | ? | ? | ? |
| Estriol | 10–15 | <1 | <1 | <1 | <1 | <0.1 | <0.1 |
| Equilin | 40 | ? | ? | ? | ? | ? | 0 |
| Alfatradiol | 15 | <1 | <1 | <1 | <1 | ? | ? |
| Epiestriol | 20 | <1 | <1 | <1 | <1 | ? | ? |
| Ethinylestradiol | 100–112 | 1–3 | 15–25 | 1–3 | <1 | 0.18 | <0.1 |
| Mestranol | 1 | ? | ? | ? | ? | <0.1 | <0.1 |
| Methylestradiol | 67 | 1–3 | 3–25 | 1–3 | <1 | ? | ? |
| Moxestrol | 12 | <0.1 | 0.8 | 3.2 | <0.1 | <0.2 | <0.1 |
| Diethylstilbestrol | ? | ? | ? | ? | ? | <0.1 | <0.1 |
| Notes: Reference CBG . Sources: See template.
| |||||||
| Estrogen | RBA (%) |
Uterine weight (%) | Uterotrophy |
LH levels (%) | RBA (%)
|
|---|---|---|---|---|---|
| Control | – | 100 | – | 100 | – |
| Estradiol (E2) | 100 | 506 ± 20 | +++ | 12–19 | 100 |
| Estrone (E1) | 11 ± 8 | 490 ± 22 | +++ | ? | 20 |
| Estriol (E3) | 10 ± 4 | 468 ± 30 | +++ | 8–18 | 3 |
| Estetrol (E4) | 0.5 ± 0.2 | ? | Inactive | ? | 1 |
| 17α-Estradiol | 4.2 ± 0.8 | ? | ? | ? | ? |
| 2-Hydroxyestradiol | 24 ± 7 | 285 ± 8 | +b | 31–61 | 28 |
| 2-Methoxyestradiol | 0.05 ± 0.04 | 101 | Inactive | ? | 130 |
| 4-Hydroxyestradiol | 45 ± 12 | ? | ? | ? | ? |
| 4-Methoxyestradiol | 1.3 ± 0.2 | 260 | ++ | ? | 9 |
| 4-Fluoroestradiola | 180 ± 43 | ? | +++ | ? | ? |
| 2-Hydroxyestrone | 1.9 ± 0.8 | 130 ± 9 | Inactive | 110–142 | 8 |
| 2-Methoxyestrone | 0.01 ± 0.00 | 103 ± 7 | Inactive | 95–100 | 120 |
| 4-Hydroxyestrone | 11 ± 4 | 351 | ++ | 21–50 | 35 |
| 4-Methoxyestrone | 0.13 ± 0.04 | 338 | ++ | 65–92 | 12 |
| 16α-Hydroxyestrone | 2.8 ± 1.0 | 552 ± 42 | +++ | 7–24 | <0.5 |
| 2-Hydroxyestriol | 0.9 ± 0.3 | 302 | +b | ? | ? |
| 2-Methoxyestriol | 0.01 ± 0.00 | ? | Inactive | ? | 4 |
| Notes: Values are mean ± SD or range. ER RBA = endogenous ). b = Atypical uterotrophic effect which plateaus within 48 hours (estradiol's uterotrophy continues linearly up to 72 hours). Sources: See template.
| |||||
Differences from other estrogens
Estetrol has potent
Estetrol has a low estrogenic effect in breast/mammary gland, and when administered in combination with estradiol,
Estetrol has relatively minimal effects on liver function.
Estetrol has potent estrogenic effects in the brain in terms of relief of hot flashes,
| Estrogen | HF |
VE | UCa | FSH | LH | HDL-C | SHBG | CBG |
AGT |
Liver |
|---|---|---|---|---|---|---|---|---|---|---|
| Estradiol | 1.0 | 1.0 | 1.0 | 1.0 | 1.0 | 1.0 | 1.0 | 1.0 | 1.0 | 1.0 |
| Estrone | ? | ? | ? | 0.3 | 0.3 | ? | ? | ? | ? | ? |
| Estriol | 0.3 | 0.3 | 0.1 | 0.3 | 0.3 | 0.2 | ? | ? | ? | 0.67 |
| Estrone sulfate | ? | 0.9 | 0.9 | 0.8–0.9 | 0.9 | 0.5 | 0.9 | 0.5–0.7 | 1.4–1.5 | 0.56–1.7 |
| Conjugated estrogens | 1.2 | 1.5 | 2.0 | 1.1–1.3 | 1.0 | 1.5 | 3.0–3.2 | 1.3–1.5 | 5.0 | 1.3–4.5 |
Equilin sulfate |
? | ? | 1.0 | ? | ? | 6.0 | 7.5 | 6.0 | 7.5 | ? |
| Ethinylestradiol | 120 | 150 | 400 | 60–150 | 100 | 400 | 500–600 | 500–600 | 350 | 2.9–5.0 |
| Diethylstilbestrol | ? | ? | ? | 2.9–3.4 | ? | ? | 26–28 | 25–37 | 20 | 5.7–7.5 |
Sources and footnotes
Notes: Values are ratios, with estradiol as standard (i.e., 1.0). Abbreviations: HF = Clinical relief of liver proteins. Liver = Ratio of liver estrogenic effects to general/systemic estrogenic effects (hot flashes/gonadotropins ). Sources: See template. | ||||||||||
Antigonadotropic effects
Administration of single doses of estetrol to postmenopausal women strongly suppressed
Pharmacokinetics

The oral bioavailability of estetrol in rats was 70% relative to
Estetrol is
Chemistry
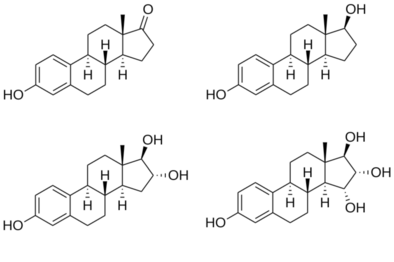
Structures of major endogenous estrogens
hydroxyl (–OH) groups : estrone (E1) has one, estradiol (E2) has two, estriol (E3) has three, and estetrol (E4) has four. |
Estetrol, also known as 15α-hydroxyestriol or as estra-1,3,5(10)-triene-3,15α,16α,17β-tetrol, is a
Synthesis
Chemical syntheses of estetrol have been published.[35]
History
Estetrol was discovered in 1965 by Egon Diczfalusy and coworkers at the Karolinska Institute in Stockholm, Sweden, via isolation from the urine of pregnant women.[2][13] Basic research on estetrol was conducted from 1965 to 1984.[2][3] It was established that estetrol is exclusively synthesized in the human fetal liver. In 1984, estetrol was regarded as a weak estrogen, which hampered its interest, and further research was virtually abandoned.[2][3] Subsequently, in 2001 Pantarhei Bioscience re-started to investigate estetrol using state-of-the-art technologies, with the sole reasoning that estetrol must have some biological role or function of importance as it would not be produced in such high quantities in the fetus otherwise.[2] By 2008, estetrol was of major interest for potential clinical use, and development was in-progress.[2][3] As of 2020, the phase III clinical development (in combination with drospirenone) for hormonal contraception has been completed[36][37] and it is in mid- to late-stage clinical development for a variety of other indications.[8] including menopausal hormone therapy (MHT) by Mithra Pharmaceuticals and advanced breast and prostate cancer by Pantarhei Oncology.
Society and culture
Legal status
Estetrol 15 mg in combination with drospirenone 3 mg has been approved for the use of hormonal contraception in Europe,[38][39] the US,[40] Canada[41] and Australia[42] and is pending approval in other countries.
Generic names
Estetrol is the
Research
Estetrol is under development for use alone for a variety of indications. Applications include
In addition to a single-drug formulation, estetrol is being developed in combination with the
Estetrol has been studied in humans at
Overdose
High single doses of estetrol of 1000 mg have been studied in women and were found to be well-tolerated.[4] Estetrol is 10 to 20 times less potent orally than the highly potent estrogen ethinylestradiol.[4] During pregnancy, estetrol levels increase to high concentrations of about 723 pg/mL on average in the mother and about 9,034 pg/mL on average in the fetus (measured via umbilical cord blood) by term.[47] Estetrol levels are 10 to 20 times higher in the fetal circulation than in the maternal circulation (which is a consequence of the fact that estetrol is produced exclusively in the fetal liver).[4][47] The production of high amounts of estetrol during pregnancy suggests that it may be a reasonably safe compound at such concentrations.[34]
Interactions
Estetrol shows minimal to no
See also
References
- ^ "Updates to the Prescribing Medicines in Pregnancy database". Therapeutic Goods Administration (TGA). 21 December 2022. Retrieved 2 January 2023.
- ^ S2CID 24003341.
- ^ S2CID 32081001.
- ^ S2CID 23568599.
- ^ S2CID 22715507.
- ^ PMID 26212489.)
{{cite journal}}: CS1 maint: DOI inactive as of January 2024 (link - ^ a b "Estetrol - Mithra Pharmaceuticals - AdisInsight".
- ^ a b c d "Drospirenone/estetrol - Mithra Pharmaceuticals". AdisInsight. Springer Nature Switzerland AG.
- ^ ISSN 1569-9056.
- ^ PMID 25359896.
- PMID 25214462.
- ^ a b Foidart, JM; et al. (2019). "30th Annual Meeting of The North America Menopause Society September 25 – 28, 2019, Chicago, IL". Menopause. 26 (12): 1445–1481. doi:10.1097/GME.0000000000001456. ISSN 1530-0374
- ^ PMID 14303250.
- ^ PMID 27451327.
- S2CID 25111733.
- ^ a b Clinical trial number NCT04209543 for "Estetra. (2020) Estetrol for the Treatment of Moderate to Severe Vasomotor Symptoms in Postmenopausal Women (E4Comfort Study I)." at ClinicalTrials.gov
- ^ a b Clinical trial number NCT04090957 for "Estetra. (2019) Estetrol for the Treatment of Moderate to Severe Vasomotor Symptoms in Postmenopausal Women (E4Comfort)." at ClinicalTrials.gov
- PMID 34956081.
- S2CID 224821517.
- S2CID 246651102.
Moreover, the introduction of other new natural oestrogenic components, such as estetrol (E4) [12], could have a similar lower VTE impact; however, we will likely need another decade to obtain results from post-marketing studies.
- ^ S2CID 11027782.
- ^ PMID 26056044.
- S2CID 35660932.
- S2CID 205931204.
- PMID 27593335.
- ^ S2CID 24616324.
- S2CID 221843478.
- S2CID 21359519.
- S2CID 32178988.
- ^ PMID 25214462.
- ^ PMID 26394847.)
{{cite journal}}: CS1 maint: DOI inactive as of January 2024 (link - ^ PMID 29931320.
- S2CID 23568599.
- ^ S2CID 28007341.
- S2CID 42017011.
- ^ Clinical trial number NCT02817841 for "E4 FREEDOM (Female Response Concerning Efficacy and Safety of Estetrol/Drospirenone as Oral Contraceptive in a Multicentric Study) - United States/Canada Study" at ClinicalTrials.gov
- ^ Clinical trial number NCT02817828 NCT02817828 for "Estetra. (2019) E4 FREEDOM (Female Response Concerning Efficacy and Safety of Estetrol/Drospirenone as Oral Contraceptive in a Multicentric Study) - EU/Russia Study." at ClinicalTrials.gov
- ^ "Drovelis EMEA authorisation". European Medicines Agency (EMA). Retrieved 4 November 2021.
- ^ "Lydisilka EMEA authorisation". European Medicines Agency (EMA). Retrieved 4 November 2021.
- ^ "Nextstellis Approval FDA". U.S. Food & Drug Administration (FDA). Retrieved 4 November 2021.
- ^ "Nextstellis Approval Health Canada". Health Canada, Government of Canada. 25 April 2012. Retrieved 4 November 2021.
- ^ "Nexstellis Approval ARTG". Australian Government, Department of Health. Retrieved 6 June 2022.[permanent dead link]
- ^ "Essential Medicines and Health Products Information Portal" (PDF).[dead link]
- ^ "News". Mithra. Archived from the original on October 1, 2015. Retrieved 2020-11-10.
- S2CID 6954183.
- S2CID 19936032.
- ^ S2CID 20399632.
Further reading
- Sakamoto H, Ohtani K, Satoh K (March 1995). "[Estetrol (E4)]". Nihon Rinsho. Japanese Journal of Clinical Medicine (in Japanese). 53 (Su Pt 2): 566–568. PMID 8753305.
- Holinka CF, Diczfalusy E, Coelingh Bennink HJ (May 2008). "Estetrol: a unique steroid in human pregnancy". The Journal of Steroid Biochemistry and Molecular Biology. 110 (1–2): 138–143. S2CID 28007341.
- Coelingh Bennink HJ, Holinka CF, Diczfalusy E (2008). "Estetrol review: profile and potential clinical applications". Climacteric. 11 (Suppl 1): 47–58. S2CID 24003341.
- Warmerdam EG, Visser M, Coelingh Bennink HJ, Groen M (2008). "A new route of synthesis of estetrol". Climacteric. 11 (Suppl 1): 59–63. S2CID 42017011.
- Visser M, Foidart JM, Coelingh Bennink HJ (2008). "In vitro effects of estetrol on receptor binding, drug targets and human liver cell metabolism". Climacteric. 11 (Suppl 1): 64–68. S2CID 11027782.
- Coelingh Bennink F, Holinka CF, Visser M, Coelingh Bennink HJ (2008). "Maternal and fetal estetrol levels during pregnancy". Climacteric. 11 (Suppl 1): 69–72. S2CID 20399632.
- Visser M, Coelingh Bennink HJ (March 2009). "Clinical applications for estetrol". The Journal of Steroid Biochemistry and Molecular Biology. 114 (1–2): 85–89. S2CID 32081001.
- Krøll J (April 2014). "Estetrol, molecular chaperones, and the epigenetics of longevity and cancer resistance". Rejuvenation Research. 17 (2): 157–158. PMID 23992378.
External links
- "Estetrol". Drug Information Portal. U.S. National Library of Medicine.