Non-mevalonate pathway
The non-mevalonate pathway—also appearing as the mevalonate-independent pathway and the 2-C-methyl-D-erythritol 4-phosphate/1-deoxy-D-xylulose 5-phosphate (MEP/DOXP) pathway—is an alternative metabolic pathway for the biosynthesis of the isoprenoid precursors isopentenyl pyrophosphate (IPP) and dimethylallyl pyrophosphate (DMAPP).[1][2][3] The currently preferred name for this pathway is the MEP pathway, since MEP is the first committed metabolite on the route to IPP.
Isoprenoid precursor biosynthesis
The mevalonate pathway (MVA pathway or HMG-CoA reductase pathway) and the MEP pathway are metabolic pathways for the biosynthesis of isoprenoid precursors: IPP and DMAPP. Whereas plants use both MVA and MEP pathway, most organisms only use one of the pathways for the biosynthesis of isoprenoid precursors. In plant cells IPP/DMAPP biosynthesis via the MEP pathway takes place in plastid organelles, while the biosynthesis via the MVA pathway takes place in the cytoplasm.[4] Most gram-negative bacteria, the photosynthetic cyanobacteria and green algae use only the MEP pathway.[5] Bacteria that use the MEP pathway include important pathogens such Mycobacterium tuberculosis.[6]
IPP and DMAPP serve as precursors for the biosynthesis of
Bacteria such as
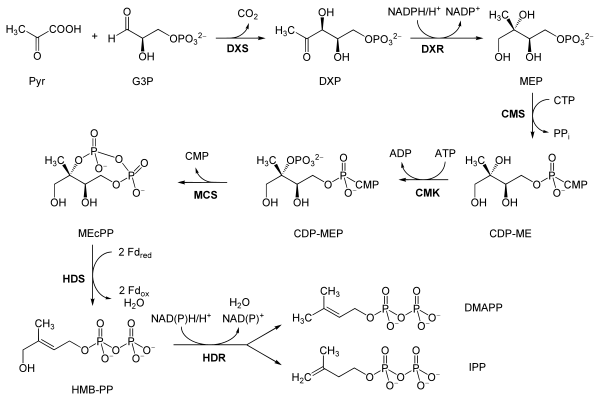
Reactions
The reactions of the MEP pathway are as follows, taken primarily from Eisenreich and co-workers, except where the bold labels are additional local abbreviations to assist in connecting the table to the scheme above:[10][9]
| Reactants | Enzyme | Product | |
|---|---|---|---|
Pyruvate (Pyr) and glyceraldehyde 3-phosphate (G3P) |
DOXP synthase (Dxs; DXP) |
1-Deoxy-D-xylulose 5-phosphate (DOXP; DXP) |  |
| DOXP (DXP) | DXP reductoisomerase (Dxr, IspC; DXR) | 2-C-methylerythritol 4-phosphate (MEP) |
 |
| MEP | 2-C-methyl-D-erythritol 4-phosphate cytidylyltransferase (YgbP, IspD; CMS) | 4-diphosphocytidyl-2-C-methylerythritol (CDP-ME) |
 |
| CDP-ME | 4-diphosphocytidyl-2-C-methyl-D-erythritol kinase (YchB, IspE; CMK) |
4-diphosphocytidyl-2-C-methyl-D-erythritol 2-phosphate (CDP-MEP) |
|
| CDP-MEP | 2-C-methyl-D-erythritol 2,4-cyclodiphosphate synthase (YgbB, IspF; MCS) | 2-C-methyl-D-erythritol 2,4-cyclodiphosphate (MEcPP) |
 |
| MEcPP | HMB-PP synthase (GcpE, IspG; HDS) |
(E)-4-Hydroxy-3-methyl-but-2-enyl pyrophosphate (HMB-PP) |  |
| HMB-PP | HMB-PP reductase (LytB, IspH; HDR) |
Isopentenyl pyrophosphate (IPP) and Dimethylallyl pyrophosphate (DMAPP) |   |
Inhibition and other pathway research
Dxs, the first enzyme of the pathway, is
DXP reductoisomerase (also known as: DXR, DOXP reductoisomerase, IspC, MEP synthase), is a key enzyme in the MEP pathway. It can be inhibited by the natural product fosmidomycin, which is under study as a starting point to develop a candidate antibacterial or antimalarial drug.[13][14][15]
The intermediate,
- IspH inhibitors: non-mevalonate Metabolic pathway that is essential for most bacteria but absent in humans, making it an ideal target for antibiotic development. This pathway, called methyl-D-erythritol phosphate (MEP) or non-mevalonate pathway, is responsible for biosynthesis of isoprenoids—molecules required for cell survival in most pathogenic bacteria and hence will be helpful in most usually antibacterial resistant bacteria.[17]
Metabolic engineering of the MEP/Non-mevalonate pathway
The MEP pathway has been extensively studied and engineered
References
- PMID 10584331.
- S2CID 24558920.
- PMID 17442674.
- PMID 15012203.
- ^ PMID 23451776.
- PMID 20615187.
- S2CID 17214504.
- PMID 31843486.
- ^ PMID 35340427|...|intentional=yes}}.)
- ^ S2CID 24558920.
- PMID 23612965.
- PMID 36575800.
- .
- PMID 10477522.
- PMID 20429517.
- S2CID 9930822.
- ^ "Research team reports new class of antibiotics active against a wide range of bacteria". MDLinx. 23 December 2020. Retrieved 23 January 2023.[dead link]
- PMID 12427975.
- ^ PMID 30025762.
- ^ ISSN 1754-5706.
- ^ PMID 33489752.
- ^ ISSN 2197-4365.


