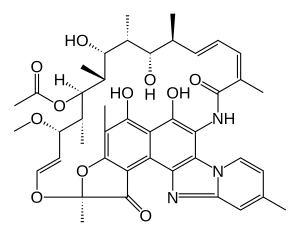
Rifaximin
 | |
 | |
| Clinical data | |
|---|---|
| Trade names | Xifaxan, Zaxine, Xifaxanta, Normix, others[1] |
| AHFS/Drugs.com | Monograph |
| MedlinePlus | a604027 |
| Pregnancy category |
|
QJ51XX01 (WHO) | |
| Legal status | |
| Legal status | |
| Pharmacokinetic data | |
| Bioavailability | < 0.4% |
| Metabolism | Liver |
| Elimination half-life | 6 hours |
| Excretion | Fecal (97%) |
| Identifiers | |
| |
JSmol) | |
| Melting point | 200 to 205 °C (392 to 401 °F) (dec.) |
| |
| |
| | |
Rifaximin, is a non-absorbable, broad spectrum antibiotic mainly used to treat travelers' diarrhea. It is based on the rifamycin antibiotics family. Since its approval in Italy in 1987, it has been licensed in over more than 30 countries for the treatment of a variety of gastrointestinal diseases like irritable bowel syndrome, and hepatic encephalopathy. It acts by inhibiting RNA synthesis in susceptible bacteria by binding to the RNA polymerase enzyme. This binding blocks translocation, which stops transcription.[4] It is marketed under the brand name Xifaxan by Salix Pharmaceuticals.[5]
Medical uses
Travelers' diarrhea
Rifaximin is used to treat travelers' diarrhea (TD) caused by E. coli bacteria in adults and children at least 12 years of age. It treats travelers' diarrhea by stopping the growth of the bacteria that cause diarrhea. Rifaximin will not work to treat travelers' diarrhea that is bloody or occurs with fever.[6]
Irritable bowel syndrome
Rifaximin is used for the treatment of
Clostridioides difficile infection
Rifaximin may also be a useful addition to
Hepatic encephalopathy
Rifaximin is used to prevent episodes of hepatic encephalopathy (changes in thinking, behavior, and personality caused by a build-up of toxins in the brain in people who have liver disease) in adults who have liver disease. It treats hepatic encephalopathy (HE) by stopping the growth of bacteria that produce toxins and that may worsen the liver disease. Although high-quality evidence is still lacking, it appears to be as effective as, or more effective than, other available treatments for hepatic encephalopathy (such as lactulose), is better tolerated, and may work faster.[10][15] It prevents reoccurring encephalopathy and is associated with high patient satisfaction. People are more compliant and satisfied to take this medication than any other due to minimal side effects, prolonged remission, and overall cost.[16] The drawbacks are increased cost, and lack of robust clinical trials for HE without combination lactulose therapy.[17]
Other uses
Other uses include treatment of:
Special caution
Patients should avoid rifaximin if they are allergic to any of rifabutin, rifampin, and rifapentine. It may cause attenuated vaccines (such as typhoid vaccine) not to work well. Health-care professionals should be informed about its usage before receiving immunization.[23] Pregnant or breastfeeding women should avoid rifaximin: it is a pregnancy category C drug and can harm the fetus.[24] Caution is required in persons with cirrhosis who have a Child–Pugh score of C.[10]
Side effects
Rifaximin has an excellent safety profile due to its lack of systemic absorption. Clinical trials did not show any serious adverse events while using the drug. There were no deaths while using it in the clinical trials.[8][9][25]
The most common side effects includes
The most serious side effects of rifaximin are:
- Clostridioides difficile-associated diarrhea (CDAD)
- Drug-resistant bacterial superinfection
- Severe allergic reactions including hives, rashes and itching
Interactions
As rifaximin is not significantly absorbed from the gut, the great majority of these drug interactions are negligible in people with healthy liver function, so healthcare providers usually do not worry about drug interactions unless liver impairment is present.[8] It may decrease the effectiveness of warfarin, a commonly prescribed anticoagulant, in people with liver problems.[27]
Pharmacology
Rifaximin is a
Mechanism of action
Rifaximin interferes with
Availability
In the United States, Salix Pharmaceuticals holds a US Patent for rifaximin and markets the drug under the name Xifaxan.
Rifaximin is approved in 33 countries for GI disorders.[37] On 13 August 2013, Health Canada issued a Notice of Compliance to Salix Pharmaceuticals Inc. for the drug product Zaxine.[38] In India, it is available under the brand names Ciboz and Xifapill.[39][40] In Russia and Ukraine the drug is sold under the name Alfa Normix (Альфа Нормикс), produced by Alfa Wassermann S.p.A. (Italy).[41] In 2018, the FDA approved a similar drug by Cosmos Pharmaceuticals called Aemcolo for traveler's diarrhea.[42]
References
- ^ "Rifaximin international". Drugs.com. 2 November 2020. Retrieved 11 November 2020.
- ^ "Product monograph brand safety updates". Health Canada. February 2024. Retrieved 24 March 2024.
- ^ "Xifaxan- rifaximin tablet". DailyMed. 1 October 2019. Retrieved 11 November 2020.
- PMID 19881343.
- ^ "Rifaximin". go.drugbank.com. Retrieved 26 June 2022.
- PMID 17582576.
- S2CID 13607138.
- ^ S2CID 25426888.
- ^ PMID 29605976.
- ^ PMID 26089696.
- PMID 17304459.
- PMID 21948965.
- PMID 28257555.
- PMID 23545528.
- .
- PMID 24672227.
- S2CID 3155213.
- PMID 36978310.
- PMID 21366632.
- PMID 26989379.
- PMID 26202193.
- PMID 23602178.
- ^ "Rifaximin Oral: Uses, Side Effects, Interactions, Pictures, Warnings & Dosing - WebMD". www.webmd.com. Retrieved 27 June 2022.
- PMID 16849349.
- PMID 32966000, retrieved 28 June 2022
- ^ "Rifaximin Side Effects". MedlinePlus. U.S. National Library of Medicine.
- S2CID 12214785.
- PMID 6526730.
- PMID 16267720.
- ^ "Rifaximin". DrugBank. 22 March 2017.
- ^ PMID 26618924.
- PMID 26576135.
- ^ "Xifaxan (Rifaximin) 550 mg - Reduce Overt Hepatic Encephalopathy Recurrences". Salix Pharmaceuticals.
- ^ a b "Product Details for NDA 022554". Orange Book: Approved Drug Products with Therapeutic Equivalence Evaluations. U.S. Food and Drug Administration (FDA).
- ^ "FDA approves two therapies to treat IBS-D" (Press release). U.S. Food and Drug Administration (FDA). Archived from the original on 26 January 2018. Retrieved 16 December 2019.
- ^ Linnane C. "Bausch Health stock soars 8.6% premarket on news of patent settlement". MarketWatch. Retrieved 14 May 2019.
- ^ "Investors | Salix Pharmaceuticals". www.salix.com.
- ^ "Summary Basis of Decision (SBD): Zaxine". Government of Canada, Health Canada, Health Products and Food Branch, Therapeutic Products Directorate, Bureau of Gastroenterology Infection and Viral Diseases. 2013.
- ^ "Brands". Zydus Healthcare Limited. Retrieved 28 June 2022.
- ^ "Trade Marks Journal" (PDF). The Government of India, Trade Mark Registry. 20 March 2017.
- ^ "Alfa Normix". Russian medical server.
- ^ "Cosmo to give Bausch Health a run for its money with FDA nod for Xifaxan rival". FiercePharma. Retrieved 14 May 2019.
