William Lipscomb
William N. Lipscomb Jr. | |
|---|---|
PhD ) | |
| Spouses | Mary Adele Sargent
(m. 1944; div. 1983)Jean Evans (m. 1983) |
| Children | 4 |
| Awards | Peter Debye Award (1973) Nobel Prize in Chemistry (1976) |
| Scientific career | |
| Fields | Nuclear magnetic resonance Theoretical chemistry Boron chemistry Biochemistry |
| Institutions | University of Minnesota Harvard University |
| Thesis | Part 1: Electron diffraction investigations of vanadium tetrachloride, dimethylketene dimer, tetrachloroethylene, and trichloroethylene Part 2: The Crystal Structure of Methylammonium Chloride (1946) |
| Doctoral advisor | Linus Pauling |
| Doctoral students | |
| Other notable students | Martha L. Ludwig Michael Rossmann Raymond C. Stevens |
William Nunn Lipscomb Jr. (December 9, 1919 – April 14, 2011)
Biography
Overview
Lipscomb was born in Cleveland, Ohio. His family moved to Lexington, Kentucky in 1920,[1] and he lived there until he received his Bachelor of Science degree in chemistry at the University of Kentucky in 1941. He went on to earn his Doctor of Philosophy degree in chemistry from the California Institute of Technology (Caltech) in 1946.
From 1946 to 1959 he taught at the
Lipscomb was married to the former Mary Adele Sargent from 1944 to 1983.[3] They had three children, one of whom lived only a few hours. He married Jean Evans in 1983.[4] They had one adopted daughter.
Lipscomb resided in Cambridge, Massachusetts until his death in 2011 from pneumonia.[5]
Early years
"My early home environment ... stressed personal responsibility and self reliance. Independence was encouraged especially in the early years when my mother taught music and when my father's medical practice occupied most of his time."
In grade school Lipscomb collected animals, insects, pets, rocks, and minerals.
Interest in astronomy led him to visitor nights at the Observatory of the University of Kentucky, where Prof. H. H. Downing gave him a copy of Baker's Astronomy. Lipscomb credits gaining many intuitive physics concepts from this book and from his conversations with Downing, who became Lipscomb's lifelong friend.
The young Lipscomb participated in other projects, such as Morse-coded messages over wires and crystal radio sets, with five nearby friends who became physicists, physicians, and an engineer.
Aged 12, Lipscomb was given a small Gilbert chemistry set. He expanded it by ordering apparatus and chemicals from suppliers and by using his father's privilege as a physician to purchase chemicals at the local drugstore at a discount. Lipscomb made his own fireworks and entertained visitors with color changes, odors, and explosions. His mother questioned his home chemistry hobby only once, when he attempted to isolate a large amount of urea from urine.
Lipscomb credits perusing the large medical texts in his physician father's library and the influence of Linus Pauling years later to his undertaking biochemical studies in his later years. Had Lipscomb become a physician like his father, he would have been the fourth physician in a row along the Lipscomb male line.
The source for this subsection, except as noted, is Lipscomb's autobiographical sketch.[6]
Education
Lipscomb's high-school chemistry teacher, Frederick Jones, gave Lipscomb his college books on organic, analytical, and general chemistry, and asked only that Lipscomb take the examinations. During the class lectures, Lipscomb in the back of the classroom did research that he thought was original (but he later found was not): the preparation of hydrogen from sodium formate (or sodium oxalate) and sodium hydroxide.[7] He took care to include gas analyses and to search for probable side reactions.
Lipscomb later had a high-school physics course and took first prize in the state contest on that subject. He also became very interested in special relativity.
Lipscomb attended University of Kentucky on a music scholarship. Prof. Robert H. Baker suggested that Lipscomb research the direct preparation of derivatives of alcohols from dilute aqueous solution without first separating the alcohol and water, which led to Lipscomb's first publication.[8]
For graduate school Lipscomb chose Caltech, which offered him a teaching assistantship in Physics at $20/month. He turned down more money from Northwestern University, which offered a research assistantship at $150/month. Columbia University rejected Lipscomb's application in a letter written by Nobel prizewinner Prof. Harold Urey.
At Caltech Lipscomb intended to study theoretical
The source for this subsection, except as noted, is Lipscomb's autobiographical sketch.[6]
Scientific studies
Lipscomb worked in three main areas, nuclear magnetic resonance and the chemical shift, boron chemistry and the nature of the chemical bond, and large biochemical molecules. These areas overlap in time and share some scientific techniques. In at least the first two of these areas Lipscomb gave himself a big challenge likely to fail, and then plotted a course of intermediate goals.
Nuclear magnetic resonance and the chemical shift

In this area Lipscomb proposed that: "... progress in structure determination, for new polyborane species and for substituted
Lipscomb investigated, "... the carboranes, C2B10H12, and the sites of electrophilic attack on these compounds[10] using nuclear magnetic resonance (NMR) spectroscopy. This work led to [Lipscomb's publication of a comprehensive] theory of chemical shifts.[11] The calculations provided the first accurate values for the constants that describe the behavior of several types of molecules in magnetic or electric fields."[12]
Much of this work is summarized in a book by Gareth Eaton and William Lipscomb, NMR Studies of Boron Hydrides and Related Compounds,[13] one of Lipscomb's two books.
Boron chemistry and the nature of the chemical bond
In this area Lipscomb originally intended a more ambitious project: "My original intention in the late 1940s was to spend a few years understanding the

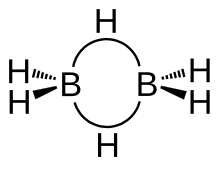
The three-center two-electron bond is illustrated in diborane (diagrams at right). In an ordinary covalent bond a pair of electrons bonds two atoms together, one at either end of the bond, the diboare B-H bonds for example at the left and right in the illustrations. In three-center two-electron bond a pair of electrons bonds three atoms (a boron atom at either end and a hydrogen atom in the middle), the diborane B-H-B bonds for example at the top and bottom of the illustrations.
Lipscomb's group did not propose or discover the three-center two-electron bond, nor did they develop formulas that give the proposed mechanism. In 1943,
The Eberhardt, Crawford, and Lipscomb paper[23] discussed above also devised the "styx number" method to catalog certain kinds of boron-hydride bonding configurations.

Wandering atoms was a puzzle solved by Lipscomb[25] in one of his few papers with no co-authors. Compounds of boron and hydrogen tend to form closed cage structures. Sometimes the atoms at the vertices of these cages move substantial distances with respect to each other. The diamond-square-diamond mechanism (diagram at left) was suggested by Lipscomb to explain this rearrangement of vertices. Following along in the diagram at left for example in the faces shaded in blue, a pair of triangular faces has a left-right diamond shape. First, the bond common to these adjacent triangles breaks, forming a square, and then the square collapses back to an up-down diamond shape by bonding the atoms that were not bonded before. Other researchers have discovered more about these rearrangements.[26] [27]
The B10H16 structure (diagram at right) determined by Grimes, Wang, Lewin, and Lipscomb found a bond directly between two boron atoms without terminal hydrogens, a feature not previously seen in other boron hydrides.[28]
Lipscomb's group developed calculation methods, both empirical[13] and from quantum mechanical theory.[29][30] Calculations by these methods produced accurate Hartree–Fock self-consistent field (SCF) molecular orbitals and were used to study boranes and carboranes.

The ethane barrier to rotation (diagram at left) was first calculated accurately by Pitzer and Lipscomb[31] using the Hartree–Fock (SCF) method.
Lipscomb's calculations continued to a detailed examination of partial bonding through "... theoretical studies of multicentered chemical bonds including both delocalized and localized molecular orbitals."[9] This included "... proposed molecular orbital descriptions in which the bonding electrons are delocalized over the whole molecule."[32]
"Lipscomb and his coworkers developed the idea of transferability of atomic properties, by which approximate theories for complex molecules are developed from more exact calculations for simpler but chemically related molecules,..."[32]
Subsequent Nobel Prize winner Roald Hoffmann was a doctoral student [33] [34] [35] [36] [37] in Lipscomb's laboratory. Under Lipscomb's direction the Extended Hückel method of molecular orbital calculation was developed by Lawrence Lohr[14] and by Roald Hoffmann.[34][38] This method was later extended by Hoffman.[39] In Lipscomb's laboratory this method was reconciled with self-consistent field (SCF) theory by Newton[40] and by Boer.[41]
Noted boron chemist M. Frederick Hawthorne conducted early[42][43] and continuing[44][45] research with Lipscomb.
Much of this work is summarized in a book by Lipscomb, Boron Hydrides,[38] one of Lipscomb's two books.
The 1976 Nobel Prize in Chemistry was awarded to Lipscomb "for his studies on the structure of boranes illuminating problems of chemical bonding".[46] In a way this continued work on the nature of the chemical bond by his doctoral advisor at the California Institute of Technology, Linus Pauling, who was awarded the 1954 Nobel Prize in Chemistry "for his research into the nature of the chemical bond and its application to the elucidation of the structure of complex substances."[47]
The source for about half of this section is Lipscomb's Nobel Lecture.[9][14]
Large biological molecule structure and function
Lipscomb's later research focused on the atomic structure of
The images below are of Lipscomb's structures from the Protein Data Bank[48] displayed in simplified form with atomic detail suppressed. Proteins are chains of amino acids, and the continuous ribbon shows the trace of the chain with, for example, several amino acids for each turn of a helix.

Carboxypeptidase A[49] (left) was the first protein structure from Lipscomb's group. Carboxypeptidase A is a digestive enzyme, a protein that digests other proteins. It is made in the pancreas and transported in inactive form to the intestines where it is activated. Carboxypeptidase A digests by chopping off certain amino acids one-by-one from one end of a protein. The size of this structure was ambitious. Carboxypeptidase A was a much larger molecule than anything solved previously.

Aspartate carbamoyltransferase.[50] (right) was the second protein structure from Lipscomb's group. For a copy of DNA to be made, a duplicate set of its nucleotides is required. Aspartate carbamoyltransferase performs a step in building the pyrimidine nucleotides (cytosine and thymidine). Aspartate carbamoyltransferase also ensures that just the right amount of pyrimidine nucleotides is available, as activator and inhibitor molecules attach to aspartate carbamoyltransferase to speed it up and to slow it down. Aspartate carbamoyltransferase is a complex of twelve molecules. Six large catalytic molecules in the interior do the work, and six small regulatory molecules on the outside control how fast the catalytic units work. The size of this structure was ambitious. Aspartate carbamoyltransferase was a much larger molecule than anything solved previously.

Leucine aminopeptidase,[51] (left) a little like carboxypeptidase A, chops off certain amino acids one-by-one from one end of a protein or peptide.

HaeIII methyltransferase[52] (right) binds to DNA where it methylates (adds a methy group to) it.

Human interferon beta[53] (left) is released by lymphocytes in response to pathogens to trigger the immune system.

Chorismate mutase[54] (right) catalyzes (speeds up) the production of the amino acids phenylalanine and tyrosine.

Fructose-1,6-bisphosphatase[55] (left) and its inhibitor MB06322 (CS-917)[56] were studied by Lipscomb's group in a collaboration, which included Metabasis Therapeutics, Inc., acquired by
Lipscomb's group also contributed to an understanding of concanavalin A[58] (low resolution structure), glucagon,[59] and carbonic anhydrase[60] (theoretical studies).
Subsequent Nobel Prize winner Thomas A. Steitz was a doctoral student in Lipscomb's laboratory. Under Lipscomb's direction, after the training task of determining the structure of the small molecule methyl ethylene phosphate,[61] Steitz made contributions to determining the atomic structures of carboxypeptidase A [49] [62] [63] [64] [65] [66] [67] [68] and aspartate carbamoyltransferase. [69] Steitz was awarded the 2009
Subsequent
Other results

The mineral lipscombite (picture at right) was named after Professor Lipscomb by the mineralogist John Gruner who first made it artificially.
Low-temperature x-ray diffraction was pioneered in Lipscomb's laboratory
Other important compounds were studied by Lipscomb and his students. Among these are hydrazine,[75] nitric oxide,[76] metal-dithiolene complexes,[77] methyl ethylene phosphate,[61] mercury amides,[78] (NO)2,[79] crystalline hydrogen fluoride,[80] Roussin's black salt,[81] (PCF3)5,[82] complexes of cyclo-octatetraene with iron tricarbonyl,[83] and leurocristine (Vincristine),[84] which is used in several cancer therapies.
Positions, awards and honors
- Guggenheim Fellow, 1954[85]
- Fellow of the American Academy of Arts and Sciences in 1960.[86]
- Member of United States National Academy of Sciences
- Member of the Faculty Advisory Board of MIT-Harvard Research Journal
- Foreign Member of the Royal Netherlands Academy of Arts and Sciences (1976)[87]
- Nobel Prize in Chemistry (1976)
Five books and published symposia are dedicated to Lipscomb.[6][88][89][90][91]
A complete list of Lipscomb's awards and honors is in his Curriculum Vitae.[92]
References
- ^ a b c d e William Lipscomb on Nobelprize.org , accessed 30 May 2020
- ^ Rifkin, Glenn (2011-04-15). "William Lipscomb, Nobel Winner in Chemistry, Dies at 91". The New York Times.
- ^ LorraineGilmer02 (2007-09-27). "obit fyi – Mary Adele Sargent Lipscomb, 1923 Ca. – 2007 NC – Sargent – Family History & Genealogy Message Board – Ancestry.com". Boards.ancestry.com. Retrieved 2012-02-01.
{{cite web}}: CS1 maint: numeric names: authors list (link) - ^ Maugh II, Thomas H. (2011-04-16). "OBITUARY: William N. Lipscomb dies at 91; won Nobel Prize in chemistry – Los Angeles Times". Articles.latimes.com. Retrieved 2012-02-01.
- ^ Kauffman, George B.; Jean-Pierre Adloff (19 July 2011). "William Nunn Lipscomb Jr. (1919–2011), Nobel Laureate and Borane Chemistry Pioneer: An Obituary–Tribute" (PDF). The Chemical Educator. 16: 195–201. Retrieved 16 August 2011.
- ^ a b c Structures and Mechanisms: From Ashes to Enzymes (Acs Symposium Series) Gareth R. Eaton (Editor), Don C. Wiley (Editor), Oleg Jardetzky (Editor), .American Chemical Society, Washington, D.C., 2002 ("Process of Discovery (1977); An Autobiographical Sketch" by William Lipscomb, 14 pp. (Lipscombite: p. xvii), and Chapter 1: "The Landscape and the Horizon. An Introduction to the Science of William N. Lipscomb", by Gareth Eaton, 16 pp.) These chapters are online at pubs.acs.org. Click PDF symbols at right.
- ^ "HighSchool – Publications – Lipscomb". Wlipscomb.tripod.com. 1937-02-25. Retrieved 2012-02-01.
- .
- ^ S2CID 46658615.
- .
- ^ Lipscomb WN, The chemical shift and other second-order magnetic and electric properties of small molecules. Advances in Nuclear Magnetic Resonance. Edited by J. Waugh, Vol. 2 (Academic Press, 1966), pp. 137-176
- ^ Hutchinson Dictionary of Scientific Biography, Lipscomb, William Nunn (1919-) (5 paragraphs) © RM, 2011, all rights reserved, as published under license in AccessScience, The McGraw-Hill Encyclopedia of Science & Technology Online, © The McGraw-Hill Companies, 2000–2008. Helicon Publishing is a division of RM. To see this biography (1) Go to accessscience.com (2) Search for Lipscomb (3) at right Click on "Lipscomb, William Nunn (1919- ). (4) If no institutional access is available, then at right click on Purchase Now (price in 2011 is about $30 US including tax for 24 hours). (5) Log in (6) Repeat steps 2 and 3.to see Lipscomb's biography.
- ^ a b Eaton GR, Lipscomb, WN. 1969. NMR Studies of Boron Hydrides and Related Compounds. W. A. Benjamin, Inc.
- ^ a b c Lipscomb WN. 1977. The Boranes and Their Relatives. in Les Prix Nobel en 1976. Imprimerie Royal PA Norstedt & Soner, Stockholm. 110-131.[1][2] Quote in next to last paragraph, which is omitted in Science version of the paper.
- .
- .
- .
- .
- .
- S2CID 137957004.
- S2CID 98533477.
- ^ H. C. Longuet-Higgins (1953). "title unknown". J. Roy. Inst. Chem. 77: 197.
- ^ .
- .
- PMID 17839704.
- PMID 18985205.
- .
- PMID 16590861.
- .
- .
- .
- ^ .
- .
- ^ .
- .
- S2CID 95702477.
- .
- ^ a b Lipscomb WN. Boron Hydrides, W. A. Benjamin Inc., New York, 1963 (Calculation methods are in Chapter 3).
- .
- .
- .
- .
- .
- .
- .
- ^ "The Nobel Prize in Chemistry 1976". Nobelprize.org. Retrieved 2012-02-01.
- ^ "The Nobel Prize in Chemistry 1954". Nobelprize.org. Retrieved 2012-02-01.
- ^ "rcsb.org". rcsb.org. Retrieved 2012-02-01.
- ^ PMID 5719196.
- PMID 6757446.
- PMID 1548695.
- S2CID 14417486.
- PMID 9342320.
- PMID 9384560.
- PMID 2157849.
- PMID 15911772.
- ^ "ligand.com". ligand.com. Retrieved 2012-02-01.
- PMID 5288772.
- ^ Haugen, W. P.; Lipscomb, W. N. (1969). "The Crystal and Molecular Structure of the Hormone Glucagon". Acta Crystallogr. A. 25 (S185).
- ^ Liang, J .-Y ., & Lipscomb, W. N., "Substrate and Inhibitor Binding to Human Carbonic Anhydrase II: a Theoretical Study", International Workshop on Carbonic Anhydrase (Spoleto, Italy VCH Verlagsgesellschaft, 1991) pp. 50-64.
- ^ .
- PMID 16591261.
- .
- ^ Ludwig, M. L., Coppola, J. C., Hartsuck, J. A., Muirhead, H., Searl, J., Steitz, T. A. and Lipscomb, W. N., "Molecular Structure of Carboxypeptidase A at 6 A Resolution", Federation Proceedings 25, Part I, 346 (1966).
- PMC 335537.
- PMID 16591584.
- ^ Lipscomb, W. N; Ludwig, M. L.; Hartsuck, J. A.; Steitz, T. A.; Muirhead, H.; Coppola, J. C.; Reeke, G. N.; Quiocho, F. A. (1967). "Molecular Structure of Carboxypeptidase A at 2.8 A Resolution and an Isomorphous Enzyme-Substrate Complex at 6 A Resolution". Federation Proceedings. 26: 385.
- ^ Coppola, J. C., Hartsuck, J. A., Ludwig, M. L., Muirhead, H., Searl, J., Steitz, T. A. and Lipscomb, W. N., "The Low Resolution Structure of Carboxypeptidase A", Acta Crystallogr. 21, A160 (1966).
- PMID 5237487.
- .
- .
- .
- .
- PMID 15391618.
- .
- .
- .
- S2CID 84983851.
- .
- .
- .
- .
- .
- PMID 5844471.
- ^ "All Fellows: L". John Simon Guggenheim Memorial Foundation. Retrieved 15 April 2011.
- ^ "Book of Members, 1780-2010: Chapter L" (PDF). American Academy of Arts and Sciences. Retrieved 15 April 2011.
- ^ "W.N. Lipscomb". Koninklijke Nederlandse Akademie van Wetenschappen (in Dutch). Archived from the original on 7 August 2011. Retrieved 15 April 2011.
- ^ The Selected Papers of William N Lipscomb Jr.: A Legacy in Structure–Function Relationships. Jainpeng Ma (Editor), Imperial College Press. 400 pp. approx. Winter 2012. (I. C. Press) Archived 2012-04-15 at the Wayback Machine (Amazon)
- ^ Boron Science: New Technologies and Applications. Narayan Hosmane (Editor), CRC Press, 878 pp. Sept, 26, 2011. (CRC Press Archived 2012-10-15 at the Wayback Machine) (Amazon)
- ^ Proceedings of the International Symposium on Quantum Chemistry, Solid-State Theory and Molecular Dynamics, International Journal of Quantum Chemistry, Quantum Chemistry Symposium No. 25, St. Augustine, Florida, March 9–16 (1991). Ed. P.O. Lowdin, Special Eds. N.Y. Orhn, J.R. Sabin, and M.C. Zemer. Published by John Wiley and Sons. 1991.
- ^ Electron Deficient Boron and Carbon Clusters, Eds: G.A. Olah, K. Wade, and R.E. Williams. An outgrowth of the January 1989 research symposium at the Loker Hydrocarbon Research Institute on Electron Deficient Clusters. Wiley – Interscience, New York, 1989. (Dedication to "The Colonel" by F. Albert Cotton, 3 pp.)
- ^ "CV – Biog – Publications – Lipscomb". Wlipscomb.tripod.com. Retrieved 2012-02-01.
External links
- "Reflections" on Linus Pauling: Video of a talk by Lipscomb. See especially the "Linus and Me" section.
- World War 2 research in brief audio clips by Lipscomb, which include his attempt to save the life of Elizabeth Swingle. Technical description of the Swingle accident.
- Scientific Character of W. Lipscomb Curriculum Vitae, publication list, science humor, Nobel Prize scrapbook, scientific aggression, family stories, portraits, eulogy.
- William Lipscomb on Nobelprize.org
- Douglas C. Rees, "William N. Lipscomb", Biographical Memoirs of the National Academy of Sciences (2019)

