Biological pump
| Part of a series of overviews on |
| Marine life |
|---|
 |
The biological pump (or ocean carbon biological pump or marine biological carbon pump) is the ocean's biologically driven
Budget calculations of the biological carbon pump are based on the ratio between
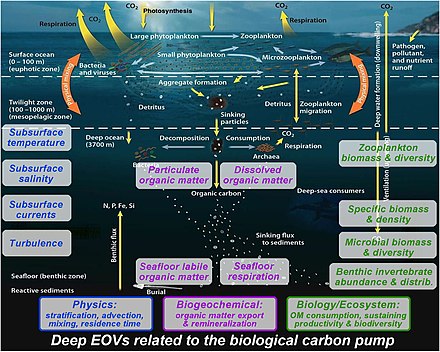
The biological pump is not so much the result of a single process, but rather the sum of a number of processes each of which can influence biological pumping. Overall, the pump transfers about 10.2
Overview

The element
The biological pump depends on the fraction of primary produced
The biological pump can be divided into three distinct phases, the first of which is the production of fixed carbon by planktonic
Once this carbon is fixed into soft or hard tissue, the organisms either stay in the euphotic zone to be recycled as part of the regenerative nutrient cycle or once they die, continue to the second phase of the biological pump and begin to sink to the ocean floor. The sinking particles will often form aggregates as they sink, greatly increasing the sinking rate. It is this aggregation that gives particles a better chance of escaping predation and decomposition in the water column and eventually making it to the sea floor.[9]
The fixed carbon that is decomposed by bacteria either on the way down or once on the sea floor then enters the final phase of the pump and is remineralized to be used again in primary production. The particles that escape these processes entirely are sequestered in the sediment and may remain there for millions of years. It is this sequestered carbon that is responsible for ultimately lowering atmospheric CO2.[9]

| Part of a series on the |
| Carbon cycle |
|---|
 |
The diagram immediately above illustrates the components of the biological pump. Biology, physics and gravity interact to pump organic carbon into the deep sea. The processes of fixation of inorganic carbon in organic matter during photosynthesis, its transformation by food web processes (trophodynamics), physical mixing, transport and gravitational settling are referred to collectively as the biological pump.[10]
The biological pump is responsible for transforming
Primary production

The first step in the biological pump is the synthesis of both organic and inorganic carbon compounds by phytoplankton in the uppermost, sunlit layers of the ocean.[13] Organic compounds in the form of sugars, carbohydrates, lipids, and proteins are synthesized during the process of photosynthesis:
CO2 + H2O + light → CH2O + O2
In addition to carbon, organic matter found in phytoplankton is composed of nitrogen, phosphorus and various
Oceanic primary production accounts for about half of the carbon fixation carried out on Earth. Approximately 50–60
Forms of carbon

Dissolved and particulate carbon
Phytoplankton supports all life in the ocean as it converts inorganic compounds into organic constituents. This autotrophically produced biomass presents the foundation of the marine food web.


Ocean carbon pools
The marine biological pump depends on a number of key pools, components and processes that influence its functioning. There are four main pools of carbon in the ocean.[5]
- Pg C [18] and includes dissolved carbon dioxide (CO2), bicarbonate (HCO−
3), carbonate (CO2−
3), and carbonic acid (H2CO3). The equilibrium between carbonic acid and carbonate determines the pH of the seawater. Carbon dioxide dissolves easily in water and its solubility is inversely related to temperature. Dissolved CO2 is taken up in the process of photosynthesis, and can reduce the partial pressure of CO2 in the seawater, favouring drawdown from the atmosphere. The reverse process respiration, releases CO2 back into the water, can increase partial pressure of CO2 in the seawater, favouring release back to the atmosphere. The formation of calcium carbonate by organisms such as coccolithophores has the effect of releasing CO2 into the water.[19][20][21][5]
- primary production, it produces around 50 Pg C y−1 globally.[29][30][31] It can be separated into living (e.g. phytoplankton, zooplankton, bacteria) and non-living (e.g. detritus) material. Of these, the phytoplankton carbon is particularly important, because of its role in marine primary production, and also because it serves as the food resource for all the larger organisms in the pelagic ecosystem.[5]
- carbonate pump, whereby PIC is exported out of the photic zone and deposited in the bottom sediments.[35][5]
Calcium carbonate

of the plates of buried coccolithophores ( see below ↓ )
Particulate inorganic carbon (PIC) usually takes the form of calcium carbonate (CaCO3), and plays a key part in the ocean carbon cycle.[36] This biologically fixed carbon is used as a protective coating for many planktonic species (coccolithophores, foraminifera) as well as larger marine organisms (mollusk shells). Calcium carbonate is also excreted at high rates during osmoregulation by fish, and can form in whiting events.[37] While this form of carbon is not directly taken from the atmospheric budget, it is formed from dissolved forms of carbonate which are in equilibrium with CO2 and then responsible for removing this carbon via sequestration.[38]
CO2 + H2O → H2CO3 → H+ + HCO3−
Ca2+ + 2HCO3− → CaCO3 + CO2 + H2O
While this process does manage to fix a large amount of carbon, two units of alkalinity are sequestered for every unit of sequestered carbon.[2][39] The formation and sinking of CaCO3 therefore drives a surface to deep alkalinity gradient which serves to raise the pH of surface waters, shifting the speciation of dissolved carbon to raise the partial pressure of dissolved CO2 in surface waters, which actually raises atmospheric levels. In addition, the burial of CaCO3 in sediments serves to lower overall oceanic alkalinity, tending to raise pH and thereby atmospheric CO2 levels if not counterbalanced by the new input of alkalinity from weathering.[1] The portion of carbon that is permanently buried at the sea floor becomes part of the geologic record. Calcium carbonate often forms remarkable deposits that can then be raised onto land through tectonic motion as in the case with the White Cliffs of Dover in Southern England. These cliffs are made almost entirely of the plates of buried coccolithophores.[40]
Oceanic carbon cycle
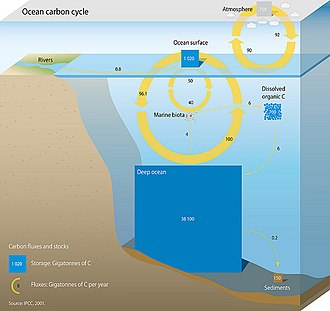
Three main processes (or pumps) that make up the marine carbon cycle bring atmospheric
Solubility pump

The biological pump is accompanied by a physico-chemical counterpart known as the solubility pump. This pump transports significant amounts of carbon in the form of dissolved inorganic carbon (DIC) from the ocean's surface to its interior. It involves physical and chemical processes only, and does not involve biological processes.[43]
The solubility pump is driven by the coincidence of two processes in the ocean:
- The solubility of carbon dioxide is a strong inverse function of seawater temperature (i.e. solubility is greater in cooler water)
- The thermohaline circulation is driven by the formation of deep water at high latitudes where seawater is usually cooler and denser
Since deep water (that is, seawater in the ocean's interior) is formed under the same surface conditions that promote carbon dioxide solubility, it contains a higher concentration of dissolved inorganic carbon than might be expected from average surface concentrations. Consequently, these two processes act together to pump carbon from the atmosphere into the ocean's interior. One consequence of this is that when deep water upwells in warmer, equatorial latitudes, it strongly outgasses carbon dioxide to the atmosphere because of the reduced solubility of the gas.[44]
Carbonate pump
The carbonate pump is sometimes referred to as the "hard tissue" component of the biological pump.[45] Some surface marine organisms, like coccolithophores, produce hard structures out of calcium carbonate, a form of particulate inorganic carbon, by fixing bicarbonate.[46] This fixation of DIC is an important part of the oceanic carbon cycle.
Ca2+ + 2 HCO3− → CaCO3 + CO2 + H2O
While the biological carbon pump fixes inorganic carbon (CO2) into
Continental shelf pump
The
Processes in the biological pump

In the diagram on the right, phytoplankton convert CO2, which has dissolved from the atmosphere into the surface oceans (90 Gt yr−1), into
The biological carbon pump is one of the chief determinants of the vertical distribution of carbon in the oceans and therefore of the surface partial pressure of CO2 governing air-sea CO2 exchange.
Marine snow
Most carbon incorporated in organic and inorganic biological matter is formed at the sea surface where it can then start sinking to the ocean floor. The deep ocean gets most of its nutrients from the higher water column when they sink down in the form of marine snow. This is made up of dead or dying animals and microbes, fecal matter, sand and other inorganic material.[63] A single phytoplankton cell has a sinking rate around one metre per day. Given that the average depth of the ocean is about four kilometres, it can take over ten years for these cells to reach the ocean floor. However, through processes such as coagulation and expulsion in predator fecal pellets, these cells form aggregates. These aggregates, known as marine snow, have sinking rates orders of magnitude greater than individual cells and complete their journey to the deep in a matter of days.[9]
In the diagram on the right,
Of the 50–60 Pg of carbon fixed annually, roughly 10% leaves the surface mixed layer of the oceans, while less than 0.5% of eventually reaches the sea floor.[9] Most is retained in regenerated production in the euphotic zone and a significant portion is remineralized in midwater processes during particle sinking. The portion of carbon that leaves the surface mixed layer of the ocean is sometimes considered "sequestered", and essentially removed from contact with the atmosphere for many centuries.[64] However, work also finds that, in regions such as the Southern Ocean, much of this carbon can quickly (within decades) come back into contact with the atmosphere.[65]
Budget calculations of the biological carbon pump are based on the ratio between
Biomineralization
Ballast minerals
| Part of a series related to |
| Biomineralization |
|---|
 |
Observations have shown that fluxes of ballast minerals (calcium carbonate, opal, and lithogenic material) and organic carbon fluxes are closely correlated in the bathypelagic zones of the ocean.[68] A large fraction of particulate organic matter occurs in the form of marine snow aggregates (>0.5 mm) composed of phytoplankton, detritus, inorganic mineral grains, and fecal pellets in the ocean.[71] Formation and sinking of these aggregates drive the biological carbon pump via export and sedimentation of organic matter from the surface mixed layer to the deep ocean and sediments. The fraction of organic matter that leaves the upper mixed layer of the ocean is, among other factors, determined by the sinking velocity and microbial remineralisation rate of these aggregates. Recent observations have shown that the fluxes of ballast minerals (calcium carbonate, opal, and lithogenic material) and the organic carbon fluxes are closely correlated in the bathypelagic zones of the ocean. This has led to the hypothesis that organic carbon export is determined by the presence of ballast minerals within settling aggregates.[72][73][74][68]
Mineral ballasting is associated with about 60% of the flux of particulate organic carbon (POC) in the high-latitude North Atlantic, and with about 40% of the flux in the Southern Ocean.[75] Strong correlations exist also in the deep ocean between the presence of ballast minerals and the flux of POC. This suggests ballast minerals enhance POC flux by increasing the sink rate of ballasted aggregates. Ballast minerals could additionally provide aggregated organic matter some protection from degradation.[76]
It has been proposed that organic carbon is better preserved in sinking particles due to increased aggregate density and sinking velocity when ballast minerals are present and/or via protection of the organic matter due to quantitative association to ballast minerals.[72][73][74] In 2002, Klaas and Archer observed that about 83% of the global particulate organic carbon (POC) fluxes were associated with carbonate, and suggested carbonate was a more efficient ballast mineral as compared to opal and terrigenous material. They hypothesized that the higher density of calcium carbonate compared to that of opal and the higher abundance of calcium carbonate relative to terrigenous material might be the reason for the efficient ballasting by calcium carbonate. However, the direct effects of ballast minerals on sinking velocity and degradation rates in sinking aggregates are still unclear.[74][68]
A 2008 study demonstrated copepod fecal pellets produced on a diet of diatoms or coccolithophorids show higher sinking velocities as compared to pellets produced on a nanoflagellate diet.
Minerals seem to enhance the flocculation of phytoplankton aggregates [78][79] and may even act as a catalyst in aggregate formation.[80] However, it has also been shown that incorporation of minerals can cause aggregates to fragment into smaller and denser aggregates.[81] This can potentially lower the sinking velocity of the aggregated organic material due to the reduced aggregate sizes, and, thus, lower the total export of organic matter. Conversely, if the incorporation of minerals increases the aggregate density, its size-specific sinking velocity may also increase, which could potentially increase the carbon export. Therefore, there is still a need for better quantitative investigations of how the interactions between minerals and organic aggregates affect the degradation and sinking velocity of the aggregates and, hence, carbon sequestration in the ocean.[81][68]
Remineralisation
For most areas of the ocean, the highest rates of carbon remineralisation occur at depths between 100–1,200 m (330–3,940 ft) in the water column, decreasing down to about 1,200 m (3,900 ft) where remineralisation rates remain pretty constant at 0.1 μmol kg−1 yr−1.[84] This provides the most nutrients available for primary producers within the photic zone, though it leaves the upper surface waters starved of inorganic nutrients.[85] Most remineralisation is done with dissolved organic carbon (DOC). Studies have shown that it is larger sinking particles that transport matter down to the sea floor[86] while suspended particles and dissolved organics are mostly consumed by remineralisation.[87] This happens in part due to the fact that organisms must typically ingest nutrients smaller than they are, often by orders of magnitude.[88] With the microbial community making up 90% of marine biomass,[89] it is particles smaller than the microbes (on the order of 10−6) that will be taken up for remineralisation.[90]
Key role of phytoplankton
| Part of a series on |
| Biogeochemical cycles |
|---|
 |
Marine phytoplankton perform half of all photosynthesis on Earth
Marine ecosystems are a major sink for atmospheric CO2 and take up similar amount of CO2 as terrestrial ecosystems, currently accounting for the removal of nearly one third of anthropogenic CO2 emissions from the atmosphere.
Passow and Carlson defined sedimentation out of the surface layer (at approximately 100 m depth) as the "export flux" and that out of the
In 2001, Hugh et al. expressed the efficiency of the biological pump as the amount of carbon exported from the surface layer (export production) divided by the total amount produced by photosynthesis (overall production).[10] Modelling studies by Buesseler and Boyd revealed that the overall transfer efficiency of the biological pump is determined by a combination of factors: seasonality;[99] the composition of phytoplankton species; the fragmentation of particles by zooplankton; and the solubilization of particles by microbes. In addition, the efficiency of the biological pump is also dependent on the aggregation and disaggregation of organic-rich aggregates and interaction between POC aggregates and suspended "ballast" minerals.[102] Ballast minerals (silicate and carbonate biominerals and dust) are the major constituents of particles that leave the ocean surface via sinking. They are typically denser than seawater and most organic matter, thus, providing a large part of the density differential needed for sinking of the particles.[72] Aggregation of particles increases vertical flux by transforming small suspended particles into larger, rapidly-sinking ones. It plays an important role in the sedimentation of phytodetritus from surface layer phytoplankton blooms.[56] As illustrated by Turner in 2015, the vertical flux of sinking particles is mainly due to a combination of fecal pellets, marine snow and direct sedimentation of phytoplankton blooms, which are typically composed of diatoms, coccolithophorids, dinoflagellates and other plankton.[56] Marine snow comprises macroscopic organic aggregates >500 μm in size and originates from clumps of aggregated phytoplankton (phytodetritus), discarded appendicularian houses, fecal matter and other miscellaneous detrital particles,[56] Appendicularians secrete mucous feeding structures or "houses" to collect food particles and discard and renew them up to 40 times a day .[103] Discarded appendicularian houses are highly abundant (thousands per m3 in surface waters) and are microbial hotspots with high concentrations of bacteria, ciliates, flagellates and phytoplankton. These discarded houses are therefore among the most important sources of aggregates directly produced by zooplankton in terms of carbon cycling potential.[104][61]

The composition of the phytoplankton community in the euphotic zone largely determines the quantity and quality of organic matter that sinks to depth.
Zooplankton grazing
Sloppy feeding

Collected from marine snow catchers (a–c) and sediment traps (d–f).
Morphological classes: (a, d) round, (b, e) cylindrical, and (c, f) ovoid.
Scale bar = 0.5 mm
In addition to linking primary producers to higher trophic levels in marine food webs, zooplankton also play an important role as "recyclers" of carbon and other nutrients that significantly impact marine biogeochemical cycles, including the biological pump. This is particularly the case with copepods and krill, and is especially important in oligotrophic waters of the open ocean. Through sloppy feeding, excretion, egestion, and leaching of fecal pellets, zooplankton release dissolved organic matter (DOM) which controls DOM cycling and supports the microbial loop. Absorption efficiency, respiration, and prey size all further complicate how zooplankton are able to transform and deliver carbon to the deep ocean.[111]
Excretion and sloppy feeding (the physical breakdown of food source) make up 80% and 20% of crustacean zooplankton-mediated DOM release respectively.[113] In the same study, fecal pellet leaching was found to be an insignificant contributor. For protozoan grazers, DOM is released primarily through excretion and egestion and gelatinous zooplankton can also release DOM through the production of mucus. Leaching of fecal pellets can extend from hours to days after initial egestion and its effects can vary depending on food concentration and quality.[114][115] Various factors can affect how much DOM is released from zooplankton individuals or populations.
Fecal pellets
The fecal pellets of zooplankton can be important vehicles for the transfer of particulate organic carbon (POC) to the deep ocean, often making large contributions to the carbon sequestration. The size distribution of the copepod community indicates high numbers of small fecal pellets are produced in the
Microbial loop
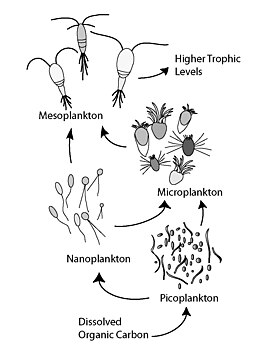
Bacterial lysis
The