Ohmefentanyl
 | |
 | |
| Clinical data | |
|---|---|
| ATC code |
|
| Legal status | |
| Legal status |
|
| Identifiers | |
| |
JSmol) | |
| |
| |
| (verify) | |
Ohmefentanyl (also known as β-hydroxy-3-methylfentanyl, OMF and RTI-4614-4)
There are eight possible
The 4″-fluoro analogue (i.e., substituted on the phenethyl ring) of the 3R,4S,βS isomer of ohmefentanyl is one of the most potent opioid agonists yet discovered, possessing an analgesic potency approximately 18,000 times that of morphine.[9] Other analogues with potency higher than that of ohmefentanyl itself include the 2′-fluoro derivative (i.e., substituted on the aniline phenyl ring), and derivatives where the N-propionyl group was replaced by N-methoxyacetyl or 2-furamide groups, or a carboethoxy group is added to the 4-position of the piperidine ring. The latter is listed as being up to 30,000 times more potent than morphine.[10]
Side effects of
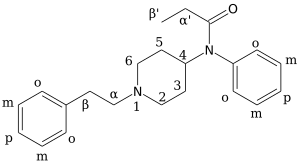


Synthesis

See also
References
- PMID 1646357.
- PMID 7739013.
- PMID 7658453.
- ^ H. D. Banks, C. P. Ferguson (September 1988). "The Metabolites of Fentanyl and its Derivatives" (PDF). U.S. Army Chemical Research, Development and Engineering Center, Aberdeen Proving Ground, MD. Archived (PDF) from the original on July 14, 2014.
- PMID 6264594.
- PMID 6679170.
- PMID 10901279.
- PMID 15051423.
- PMID 12779044.
- ISSN 0929-8673.
- PMID 25976511.
External links
- Ohmefentanyl at the U.S. National Library of Medicine Medical Subject Headings (MeSH)
