Menthol
| |||
 | |||
| Names | |||
|---|---|---|---|
| Preferred IUPAC name
5-Methyl-2-(propan-2-yl)cyclohexan-1-ol | |||
| Other names
2-Isopropyl-5-methylcyclohexan-1-ol
2-Isopropyl-5-methylcyclohexanol 3-p-Menthanol Hexahydrothymol Menthomenthol Peppermint camphor | |||
| Identifiers | |||
3D model (
JSmol ) |
|||
| ChEBI | |||
| ChEMBL | |||
| ChemSpider | |||
| DrugBank | |||
ECHA InfoCard
|
100.016.992 | ||
| EC Number |
| ||
IUPHAR/BPS |
|||
| KEGG | |||
PubChem CID
|
|||
RTECS number
|
| ||
| UNII |
| ||
CompTox Dashboard (EPA)
|
|||
| |||
SMILES
| |||
| Properties | |||
| C10H20O | |||
| Molar mass | 156.269 g·mol−1 | ||
| Appearance | White or colorless crystalline solid | ||
| Odor | mint-licorice | ||
| Density | 0.890 g·cm−3, solid (racemic or (−)-isomer) | ||
| Melting point | 36–38 °C (97–100 °F; 309–311 K) racemic 42–45 °C, (−)-isomer, α crystalline form | ||
| Boiling point | 214.6 °C (418.3 °F; 487.8 K) | ||
| Slightly soluble, (−)-isomer | |||
| Hazards[1] | |||
| Occupational safety and health (OHS/OSH): | |||
Main hazards
|
Irritant, flammable | ||
| GHS labelling: | |||

| |||
| Warning | |||
| H315, H319 | |||
| P264, P280, P302+P352, P305+P351+P338, P332+P313, P337+P313, P362 | |||
| NFPA 704 (fire diamond) | |||
| Flash point | 93 °C (199 °F; 366 K) | ||
| Safety data sheet (SDS) | External MSDS | ||
| Related compounds | |||
Related alcohols
|
Cyclohexanol, Pulegol, Dihydrocarveol, Piperitol | ||
Related compounds
|
|||
| Supplementary data page | |||
| Menthol (data page) | |||
Except where otherwise noted, data are given for materials in their standard state (at 25 °C [77 °F], 100 kPa).
| |||
Menthol is an organic compound, specifically a monoterpenoid, that occurs naturally in the oils of several plants in the mint family, such as corn mint and peppermint. It is a white or clear waxy crystalline substance that is solid at room temperature and melts slightly above. The main form of menthol occurring in nature is (−)-menthol, which is assigned the (1R,2S,5R) configuration.
For many people, menthol produces a cooling sensation when inhaled, eaten, or applied to the skin, and mint plants have been used for centuries for topical pain relief and as a food flavoring. Menthol has local anesthetic and counterirritant qualities, and it is widely used to relieve minor throat irritation.
Menthol has been demonstrated to cause a subjective nasal decongestant effect without any objective decongestant action, and administration of menthol via a nasal inhaler in humans has also been shown to cause nasal congestion.[3][4]
Menthol also acts as a weak κ-opioid receptor agonist.
Structure
Natural menthol exists as one pure
In the natural compound, the
The (+)- and (−)-enantiomers of menthol are the most stable among these based on their cyclohexane conformations. With the ring itself in a chair conformation, all three bulky groups can orient in equatorial positions.
The two crystal forms for
Biological properties


Menthol's ability to chemically trigger the cold-sensitive TRPM8 receptors in the skin is responsible for the well-known cooling sensation it provokes when inhaled, eaten, or applied to the skin.[5] In this sense, it is similar to capsaicin, the chemical responsible for the spiciness of hot chilis (which stimulates heat sensors, also without causing an actual change in temperature).
Menthol's
Some studies show that menthol acts as a
Menthol is widely used in dental care as a topical antibacterial agent, effective against several types of
Occurrence
Menthol occurs naturally in peppermint oil (along with a little menthone, the ester menthyl acetate and other compounds), obtained from Mentha × piperita (peppermint).[14] Japanese menthol also contains a small percentage of the 1-epimer neomenthol.[citation needed]
Biosynthesis
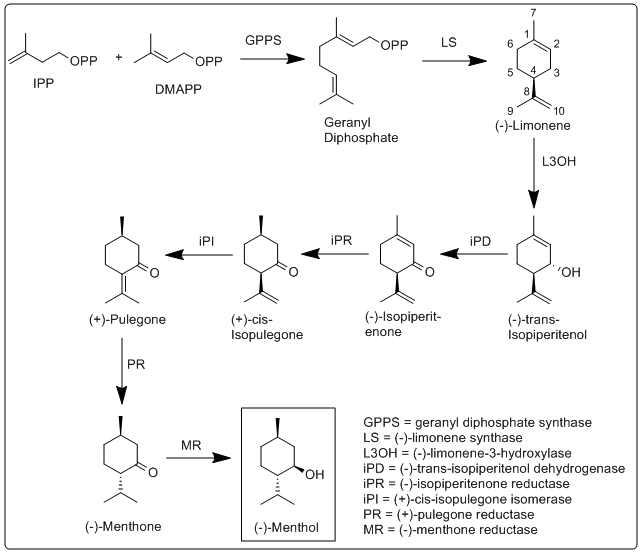
The biosynthesis of menthol has been investigated in Mentha × piperita and the enzymes involved in have been identified and characterized.[15] It begins with the synthesis of the terpene limonene, followed by hydroxylation, and then several reduction and isomerization steps.
More specifically, the biosynthesis of (−)-menthol takes place in the secretory gland cells of the peppermint plant. The steps of the biosynthetic pathway are as follows:
- geranyl diphosphate.
- (−)-limonene synthase (LS) catalyzes the cyclization of geranyl diphosphate to (−)-limonene.
- (−)-Limonene-3-hydroxylase (L3OH), using O2 and then nicotinamide adenine dinucleotide phosphate (NADPH) catalyzes the allylic hydroxylation of (−)-limonene at the 3 position to (−)-trans-isopiperitenol.
- (−)-trans-Isopiperitenol dehydrogenase (iPD) further oxidizes the hydroxyl group on the 3 position using NAD+ to make (−)-isopiperitenone.
- (−)-Isopiperitenone reductase (iPR) then reduces the double bond between carbons 1 and 2 using NADPH to form (+)-cis-isopulegone.
- (+)-cis-Isopulegone isomerase (iPI) then isomerizes the remaining double bond to form (+)-pulegone.
- (+)-Pulegone reductase (PR) reduces this double bond using NADPH to form (−)-menthone.
- (−)-Menthone reductase (MR) then reduces the carbonyl group using NADPH to form (−)-menthol.[15]
Production
Natural menthol is obtained by freezing
Total world production of menthol in 1998 was 12,000 tonnes of which 2,500 tonnes was synthetic. In 2005, the annual production of synthetic menthol was almost double. Prices are in the $10–20/kg range with peaks in the $40/kg region but have reached as high as $100/kg. In 1985, it was estimated that China produced most of the world's supply of natural menthol, although it appears that India has pushed China into second place.[16]
Menthol is manufactured as a single
The process begins by forming an
Another commercial process is the Haarmann–Reimer process (after the company Haarmann & Reimer, now part of
Applications

Menthol is included in many products, and for a variety of reasons.
Cosmetic
- In some beauty products such as hair conditioners, based on natural ingredients (e.g., St. Ives).
Medical
- As an antipruritic to reduce itching.
- As a IcyHot patches or knee/elbow sleeves.
- As a penetration enhancer in transdermal drug delivery.
- Used to cause a subjective feeling of decongestion in nasal inhalers.[5] In decongestants for chest creams and patches).
- Examples: Vicks VapoRub, Mentholatum, Axe Brand, VapoRem, Mentisan.
- In certain medications used to treat sunburns, as it provides a cooling sensation (then often associated with aloe).
- Commonly used in oral hygiene products and bad-breath remedies, such as mouthwash, toothpaste, mouth and tongue sprays, and more generally as a food flavor agent; such as in chewing gum and candy.
- In first aid products such as "mineral ice" to produce a cooling effect as a substitute for real ice in the absence of water or electricity (pouch, body patch/sleeve or cream).
- In nonprescription products for short-term relief of minor sore throat and minor mouth or throat irritation e.g.: cough medicines.
- A recent study showed improvement in Alzheimer's symptoms and cognition improvements in mice.[20]
Others
- In razor burn.
- As a smoking tobacco additive in some cigarette brands, for flavor, and to reduce throat and sinus irritation caused by smoking. Menthol also increases nicotine receptor density,[21] increasing the addictive potential of tobacco products.[22][23]
- As a pesticide against tracheal mites of honey bees.
- In perfumery, menthol is used to prepare menthyl esters to emphasize floral notes (especially rose).
- In various patches ranging from fever-reducing patches applied to children's foreheads to "foot patches" to relieve numerous ailments (the latter being much more frequent and elaborate in Asia, especially Japan: some varieties use "functional protrusions", or small bumps to massage one's feet as well as soothing them and cooling them down).
- As an upper gastrointestinal endoscopy.[24]
Organic chemistry
In
- It can be used as a catalyst for sodium production for the amateur chemist via the alcohol catalysed magnesium reduction process.[25]
- Menthol is potentially ergogenic (performance enhancing) for athletic performance in hot environments[26]
Reactions
Menthol reacts in many ways like a normal secondary alcohol. It is oxidised to
History
In the West, menthol was first isolated in 1771, by the German, Hieronymus David Gaubius.[29] Early characterizations were done by Oppenheim,[30] Beckett,[31] Moriya,[32] and Atkinson.[33] It was named by F. L. Alphons Oppenheim (1833–1877) in 1861.[34]
Compendial status
- United States Pharmacopeia 23[35][clarification needed]
- Japanese Pharmacopoeia 15[36]
- Food Chemicals Codex[37]
Safety
The estimated
Survival after doses of 8 to 9 g has been reported.[38] Overdose effects are abdominal pain, ataxia, atrial fibrillation, bradycardia, coma, dizziness, lethargy, nausea, skin rash, tremor, vomiting, and vertigo.[39]
See also
References
- ^ "l-Menthol". pubchem.ncbi.nlm.nih.gov.
- Reckitt Benckiser. 27 October 2016. Retrieved 3 August 2018.
- ISSN 1534-6315.
- ISSN 0276-0673.
- ^ S2CID 20568911.
- S2CID 33979563.
- S2CID 24596984.
- PMID 12200946.)
{{cite journal}}: CS1 maint: DOI inactive as of November 2024 (link - PMID 24460753.
- PMID 18593637.
- PMID 28769802.
- PMID 25911964.
- S2CID 11029018.
- ISBN 978-1-56363-678-3.
- ^ S2CID 206871270.
- ISBN 978-1-118-40692-2
- ^ "Japan: Takasago to Expand L-Menthol Production in Iwata Plant". Flex News Food.
- .
- ISBN 978-0-85404-824-3.[page needed]
- ^ "Unexpected Link Between Menthol and Alzheimer's Discovered in Mice". 22 October 2024.
- PMID 26961950.
- PMID 27473365.
- PMID 26339211.
- S2CID 24399887.
- ^ "Make Sodium Metal with Menthol (And a bunch of other stuff...)". YouTube. 14 February 2019.
- PMID 32623642.
- ^ Sandborn LT. "l-Menthone". Organic Syntheses; Collected Volumes, vol. 1, p. 340.
- doi:10.59720/20-058.
- ^ Adversoriorum varii argumentii. Vol. 1. Leiden. 1771. p. 99.
- .
- .
- .
- .
- ^ Oppenheim A (1861). "Note sur le camphre de menthe" [On the camphor of mint]. Comptes Rendus. 53: 379–380.
Les analogies avec le bornéol me permettent de proposer pour ce corps le nom de menthol,… [Analogies with borneol allow me to propose the name menthol for this substance,…]
- ^ Therapeutic Goods Administration (1999). "Approved Terminology for Medicines" (PDF). Archived from the original (PDF) on 22 May 2006. Retrieved 29 June 2009.
- ^ "Japanese Pharmacopoeia". Archived from the original on 9 April 2008. Retrieved 29 June 2009.
- Sigma Aldrich. "DL-Menthol". Retrieved 15 February 2022.
- ISBN 978-0-8493-1284-7
- ISBN 978-1-4200-4479-9
Further reading
- Turner EE, Harris MM (1952). Organic Chemistry. London: Longmans, Green & Co.
- Handbook of Chemistry and Physics (71st ed.). Ann Arbor, MI: CRC Press. 1990.
- The Merck Index (7th ed.). Rahway, NJ: Merck & Co. 1960.
- "Aroma Chemical Profile: Menthol". Perfumer & Flavorist. 32 (12): 38–47. December 2007.
- Colacot TJ (1 April 2002). "2001 Nobel Prize in Chemistry: Timely recognition for rhodium, ruthenium and osmium-catalysed chiral reactions". Platinum Metals Rev. 46 (2): 82–83. .
External links
- Ryoji Noyori Nobel lecture (2001)
- A review of menthol from the Science Creative Quarterly