Orphenadrine
 | |
| Clinical data | |
|---|---|
| Trade names | Generic; many brand names[1] |
| AHFS/Drugs.com | Monograph |
| MedlinePlus | a682162 |
| Pregnancy category |
|
| Routes of administration | Oral, intravenous, intramuscular |
| ATC code | |
| Legal status | |
| Legal status | |
| Pharmacokinetic data | |
| Bioavailability | 90% |
| Protein binding | 95% |
| Metabolism | Hepatic demethylation |
| Elimination half-life | 13–20 hours[2] |
| Excretion | Renal and biliary |
| Identifiers | |
| |
JSmol) | |
| |
| |
| (verify) | |
Orphenadrine (sold under many
Medical use
Orphenadrine is a skeletal muscle relaxant.
Orphenadrine and other muscle relaxants are sometimes used to treat pain arising from rheumatoid arthritis but there is no evidence they are effective for that purpose.[5]
In 2003, a
Side effects
Orphenadrine has the side effects of the other common antihistamines in large part. Stimulation is somewhat more common than with other related antihistamines, and is especially common in the elderly. Common side effects include dry mouth, dizziness, drowsiness, constipation, urine retention, blurred vision, and headache.[3] Its use in Parkinson's is especially limited by these factors.[6]
Orphenadrine is contraindicated in patients with
Continuous and/or cumulative use of anticholinergic medications, including first-generation antihistamines, is associated with higher risk of cognitive decline and dementia in older people.[9][10]
Pharmacology
Orphenadrine is known to have these pharmacological properties:
- Nonselective mACh receptor antagonist (anticholinergic, 58% as potent as atropine)[11] Various monographs and package inserts, nursing manuals, journal articles and so forth have proposed the theory that this anticholinergic (atropine-like) activity, NMDA antagonism and possible local anaesthetic and miscellaneous analgesic effects may be the reason for orphenadrine's efficacy against muscle and other pain.[12] These reasons are behind the use of orphenadrine and other drugs of a number of types which are used with paracetamol, aspirin, naproxen, and similar agents with or without opioid analgesics to more effectively manage pain of various types.[13]
- NMDA receptor antagonist[14] (Ki value of 6.0 ± 0.7 μM, one hundred times less potent than phencyclidine, which binds with a Ki of 59 nM)[15][16]
- NDRI (norepinephrine and dopamine reuptake inhibitor)[17][18]
- Nav1.7, Nav1.8, and Nav1.9 sodium channel blocker[19]
- HERG potassium channel blocker[20]
History
Prior to the development of amantadine in the late 1960s and then other drugs, anticholinergics like orphenadrine were the mainstay of Parkinson's treatment.[7]
Formulation
Orphenadrine has been available as a citrate salt and a hydrochloride salt; in the US as of February 2016 the citrate form was available in tablets, extended release tablets, compounding powder and by injection for acute use in a hospital setting.[1][22]
Orphenadrine is often available mixed with aspirin, paracetamol/acetaminophen, ibuprofen, caffeine, and/or codeine.[1]
The brand names Norflex and Norgesic are formulations of the citrate salt of orphenadrine and Disipal is the hydrochloride salt.[23]
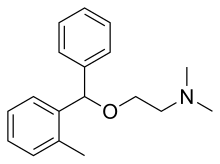
Chemistry
Orphenadrine is a
Stereochemistry
Orphenadrine has a
| Enantiomers | |
|---|---|
 (R)-orphenadrine CAS number: 33425-91-1 |
 (S)-orphenadrine CAS number: 33425-89-7 |
References
- ^ a b c d e "Orphenadrine". Drugs.com international listings. Retrieved 5 February 2016.
- S2CID 24631265.
- ^ a b c "Orphenadrine". Medline Plus. 1 December 2010. Retrieved 6 February 2016.
- PMID 15276195.
- S2CID 205197256.
- ^ PMID 12804486.
- ^ ISBN 978-0-19-261911-2.
- ^ "Orphenadrine Citrate Extended release label" (PDF). U.S. Food and Drug Administration. October 1998. Archived (PDF) from the original on 2023-10-04. Retrieved 2023-10-04.
- PMID 25621434.
- PMID 19636034.
- PMID 3353357.
- ^ Nurses' Drug Guide 2010 [full citation needed]
- ^ PMID 2578597.
- S2CID 10142765.
- S2CID 10142765.
- PMID 12232776.
- S2CID 31845784.
- PMID 26106364.
- S2CID 17830280.
- S2CID 20049051.
- ISBN 978-0-471-89979-2.
- ^ "FDA listing of Orphenadrine citrate registrations". United States Food and Drug Administration. Retrieved 6 February 2016.
- ^ "Disipal Brand of Orphenadrine HCl". Riker.
- ISBN 978-0-12-417213-5.
- ISBN 978-3-946057-10-9.
