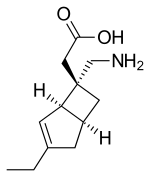
Mirogabalin
 | |
| Clinical data | |
|---|---|
| Trade names | Tarlige |
| Other names | DS-5565 |
| Routes of administration | By mouth |
| ATC code | |
| Identifiers | |
| |
JSmol) | |
| |
| |
Mirogabalin (brand name Tarlige; developmental code name DS-5565) is a gabapentinoid medication developed by Daiichi Sankyo. Gabapentin and pregabalin are also members of this class. As a gabapentinoid, mirogabalin binds to the α2δ subunit of voltage-gated calcium channel (1 and 2), but with significantly higher potency than pregabalin. It has shown promising results in Phase II clinical trials for the treatment of diabetic peripheral neuropathic pain.[1][2]
Phase III trial results:
- Effective: for post-herpetic neuralgia(trial: NEUCOURSE)
- Ineffective: for fibromyalgia (trial: ALDAY)[3]
- Effective: for diabetic peripheral neuropathic pain (trial: REDUCER)[4]
In Japan, the company submitted a marketing application for treatment of peripheral neuropathic pain.[5] The medication was approved for neuropathic pain and postherpetic neuralgia in Japan in January 2019.[6]
References
- PMID 25231896.
- ^ Vinik A, Sharma U, Feins K, Hsu C, Merante D (2014). "DS-5565 for the Treatment Of Diabetic Peripheral Neuropathic Pain: Randomized, Double-Blind, Placebo- And Active Comparator-Controlled Phase II Study (S20.004)". Neurology. 82 (10): S20.004.
- ^ "Daiichi Sankyo Announces Top-line Results from Phase 3 Global Clinical Development Program Evaluating Mirogabalin in Pain Syndromes". Daiichi Sankyo. 30 June 2017.
- ^ "Daiichi Sankyo Announces Positive Top-line Results from Phase 3 Clinical Trial Evaluating Mirogabalin in Diabetic Peripheral Neuropathic Pain". Daiichi Sankyo. 31 August 2017.
- ^ "Daiichi Sankyo Submits Marketing Application for Mirogabalin in Japan". Daiichi Sankyo. 15 February 2018.
- ^ "Mirogabalin - Daiichi Sankyo Company - AdisInsight".
External links
- "Mirogabalin". Drug Information Portal. U.S. National Library of Medicine.
