ELB-139
 | |
| Identifiers | |
|---|---|
| |
JSmol) | |
| |
| |
| (verify) | |
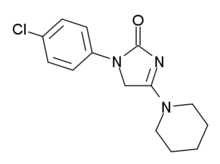
ELB-139 (LS-191,811) is an anxiolytic drug with a novel chemical structure, which is used in scientific research. It has similar effects to benzodiazepine drugs, but is structurally distinct and so is classed as a nonbenzodiazepine anxiolytic.[1][2]
ELB-139 is a subtype-selective
GABAA receptors, with highest affinity for the α3 subtype, but highest efficacy at α1 and α2.[3] It has primarily anxiolytic and anticonvulsant effects, but produces little sedative effects or ataxia,[4] and has also been demonstrated in rats to increase serotonin levels in the striatum and prefrontal cortex, without affecting dopamine levels.[5] It has been proposed as a possible candidate for a novel non-sedating anxiolytic or anticonvulsant drug for use in humans[6] The sponsor elbion AG registered a clinical trial in ClinicalTrials.gov
for the treatment of anxiety associated with panic disorder but the results have not been reported.[7] It was developed by Arzneimittelwerk Dresden in the 1990s.[8]
