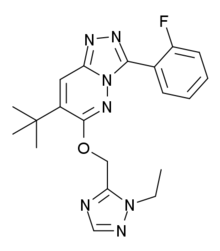
TPA-023
 | |
 | |
| Clinical data | |
|---|---|
| Other names | MK-0777 |
| Routes of administration | By mouth |
| Pharmacokinetic data | |
| Metabolism | liver |
| Elimination half-life | 6.7 hours |
| Identifiers | |
| |
JSmol) | |
| |
| |
| (verify) | |
TPA-023 (MK-0777) is an
antagonist at α1 and α5-containing subtypes.[1] It has primarily anxiolytic and anticonvulsant effects in animal tests, but with no sedative effects even at 50 times the effective anxiolytic dose.[2][3]
In human trials on healthy volunteers, TPA-023 was comparable to lorazepam, but had much less side effects on cognition, memory, alertness or coordination.[4] In Phase II trials, the compound was significantly superior to placebo without inducing sedation. The clinical development was halted due to preclinical toxicity (cataract) in long term dosing studies.[5][6] TPA-023 is well absorbed following oral administration and extensively metabolised by the liver, with a half-life of 6.7 hours.[7] The main enzyme involved in its metabolism is CYP3A4, with some contribution by CYP3A5.[8]
