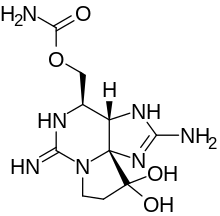
Saxitoxin
| |||
| |||
| Names | |||
|---|---|---|---|
| IUPAC name
[(3aS,4R,10aS)-10,10-dihydroxy-2,6-diiminooctahydro-1H,8H-pyrrolo[1,2-c]purin-4-yl]methyl carbamate
| |||
| Identifiers | |||
3D model (
JSmol ) |
|||
| ChEBI | |||
| ChEMBL | |||
| ChemSpider | |||
ECHA InfoCard
|
100.160.395 | ||
IUPHAR/BPS |
|||
| KEGG | |||
PubChem CID
|
|||
| UNII | |||
CompTox Dashboard (EPA)
|
|||
| |||
| |||
| Properties | |||
| C10H17N7O4 | |||
| Molar mass | 299.291 g·mol−1 | ||
Except where otherwise noted, data are given for materials in their standard state (at 25 °C [77 °F], 100 kPa).
| |||
Saxitoxin (STX) is a potent neurotoxin and the best-known paralytic shellfish toxin (PST). Ingestion of saxitoxin by humans, usually by consumption of shellfish contaminated by toxic algal blooms, is responsible for the illness known as paralytic shellfish poisoning (PSP).
The term saxitoxin originates from the genus name of the butter clam (Saxidomus) from which it was first isolated. But the term saxitoxin can also refer to the entire suite of more than 50 structurally related neurotoxins (known collectively as "saxitoxins") produced by protists, algae and cyanobacteria which includes saxitoxin itself (STX), neosaxitoxin (NSTX), gonyautoxins (GTX) and decarbamoylsaxitoxin (dcSTX).
Saxitoxin has a large environmental and economic impact, as its presence in
Source in nature
Saxitoxin is a
Saxitoxin has also been found in at least twelve marine
Structure and synthesis
Saxitoxin di
Several total syntheses of saxitoxin have been accomplished.[14][15][16]
Mechanism of action

Saxitoxin is a neurotoxin that acts as a
The voltage-gated sodium channel is essential for normal neuronal functioning. It exists as integral membrane proteins interspersed along the axon of a neuron and possessing four domains that span the cell membrane. Opening of the voltage-gated sodium channel occurs when there is a change in voltage or some ligand binds in the right way. It is of foremost importance for these sodium channels to function properly, as they are essential for the propagation of an action potential. Without this ability, the nerve cell becomes unable to transmit signals and the region of the body that it enervates is cut off from the nervous system. This may lead to paralysis of the affected region, as in the case of saxitoxin.[3]
Saxitoxin binds reversibly to the sodium channel. It binds directly in the pore of the channel protein, occluding the opening, and preventing the flow of sodium ions through the membrane. This leads to the nervous shutdown described above.[3]
Biosynthesis
Although the
Saxitoxin biosynthesis is the first non-terpene alkaloid pathway described for bacteria, though the exact mechanism of saxitoxin biosynthesis is still essentially a theoretical model. The precise mechanism of how substrates bind to enzymes is still unknown, and genes involved in the biosynthesis of saxitoxin are either putative or have only recently been identified.[19][20]
Two biosyntheses have been proposed in the past. Earlier versions differ from a more recent proposal by Kellmann, et al. based on both biosynthetic considerations as well as genetic evidence not available at the time of the first proposal. The more recent model describes a STX gene cluster (sxt) used to obtain a more favorable reaction. The most recent reaction sequence of Sxt in cyanobacteria[20] is as follows. Refer to the diagram for a detailed biosynthesis and intermediate structures.
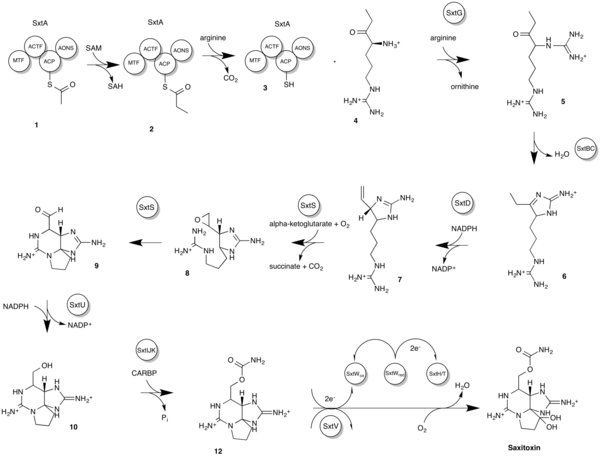
- It begins with the loading of the acyl carrier protein (ACP) with acetate from acetyl-CoA, yielding intermediate 1.
- This is followed by SxtA-catalyzed methylation of acetyl-ACP, which is then converted to propionyl-ACP, yielding intermediate 2.
- Later, another SxtA performs a Claisen condensation reaction between propionyl-ACP and arginine producing intermediate 4 and intermediate 3.
- SxtG transfers an amidino group from an arginine to the α-amino group of intermediate 4 producing intermediate 5.
- Intermediate 5 then undergoes retroaldol-like condensation by SxtBC, producing intermediate 6.
- SxtD adds a double bond between C-1 and C-5 of intermediate 6, which gives rise to the 1,2-H shift between C-5 and C-6 in intermediate 7.
- SxtS performs an epoxidation of the double bond yielding intermediate 8, and then an opening of the epoxide to an aldehyde, forming intermediate 9.
- SxtU reduces the terminal aldehyde group of the STX intermediate 9, thus forming intermediate 10.
- SxtIJK catalyzes the transfer of a carbamoyl group to the free hydroxyl group on intermediate 10, forming intermediate 11.
- SxtH and SxtT, in conjunction with SxtV and the SxtW gene cluster, perform a similar function which is the consecutive hydroxylation of C-12, thus producing saxitoxin and terminating the STX biosynthetic pathway.
Illness and poisoning
Toxicology
Saxitoxin is highly toxic to
Illness in humans
The human illness associated with ingestion of harmful levels of saxitoxin is known as paralytic shellfish poisoning, or PSP, and saxitoxin and its derivatives are often referred to as "PSP toxins".[1]
The medical and environmental importance of saxitoxin derives from the consumption of contaminated
Studies in animals have shown that the lethal effects of saxitoxin can be reversed with
Military interest
Saxitoxin, by virtue of its extremely low
After the 1969 ban on
It is listed in
See also
- Canadian Reference Materials
- Action potential – Neuron communication by electric impulses
- Alexandrium tamarense – Species of single-celled organism
- Anabaena circinalis – Species of bacterium
- Harmful algal bloom – Population explosion of organisms that can kill marine life
- Paralytic shellfish poisoning – Syndrome of shellfish poisoning
- Brevetoxin – Class of chemical compounds produced naturally
- Ciguatoxin – Group of chemical compounds
- Domoic acid – chemical compound
- Okadaic acid – chemical compound
- Tetrodotoxin – Neurotoxin
References
- ^ PMID 10485519. Archived from the original on October 7, 2008. Retrieved 2008-08-12.)
{{cite journal}}: CS1 maint: unfit URL (link - S2CID 86185142.
- ^ a b c d e "Saxitoxin". Retrieved April 10, 2022.
- PMID 19560484.
- PMID 11319121.
- ^ PMID 9028016.
- PMID 17035133.
- PMID 18728730.
- PMID 10414862.
- PMID 21543051. Archived from the original(PDF) on 2016-03-05. Retrieved 2022-04-10.
- PMID 1176726.
- PMID 1133383.
- PMID 621331.
- PMID 850038.
- PMID 22098556.
- PMID 17658800.
- )
- PMID 2542373.
- ^ PMID 21625593.
- ^ PMID 18487408.
- ISSN 1057-9419. Retrieved 26 May 2012.
- ^
Benton BJ, Keller SA, Spriggs DL, Capacio BR, Chang FC (1998). "Recovery from the lethal effects of saxitoxin: A therapeutic window for 4-aminopyridine (4-AP)". Toxicon. 36 (4): 571–588. PMID 9643470.
- ^
Chang FC, Spriggs DL, Benton BJ, Keller SA, Capacio BR (1997). "4-Aminopyridine reverses saxitoxin (STX)- and tetrodotoxin (TTX)-induced cardiorespiratory depression in chronically instrumented guinea pigs". Fundamental and Applied Toxicology. 38 (1): 75–88. S2CID 17185707.
- ^
Chen H, Lin C, Wang T (1996). "Effects of 4-Aminopyridine on Saxitoxin Intoxication". Toxicology and Applied Pharmacology. 141 (1): 44–48. PMID 8917674.
- ^ "Paralytic shellfish poisoning (PSP)" (PDF). Fish Dept. Sabah Malaysia. Archived from the original (PDF) on October 25, 2021. Retrieved April 10, 2022.
- ISBN 978-0-7637-2425-2. Retrieved 4 May 2015.
- ^ ISBN 978-0-674-01699-6. Retrieved 4 May 2015.
- )
- ^ "Saxitoxin fact sheet" (PDF). Organisation for the Prohibition of Chemical Weapons (OPCW). June 2014.
External links
- [1] Paralytic Shellfish Poisoning
- [2] Neil Edwards. The Chemical Laboratories. School of Chemistry, Physics & Environmental Science. University of Sussex at Brighton. Saxitoxin - from food poisoning to chemical warfare
- Toxic cyanobacteria in water: A guide to their public health consequences, monitoring and management. Edited by Ingrid Chorus and Jamie Bartram, 1999. Published by World Health Organization. ISBN 0-419-23930-8