Barton–McCombie deoxygenation
| Barton–McCombie deoxygenation | |
|---|---|
| Named after | Derek Harold Richard Barton Stuart W. McCombie |
| Reaction type | Organic redox reaction |
| Identifiers | |
| Organic Chemistry Portal | barton-mccombie-reaction |
| RSC ontology ID | RXNO:0000134 |
The Barton–McCombie deoxygenation is an
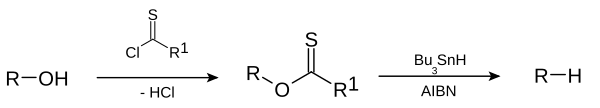
This deoxygenation reaction is a radical substitution. In the related Barton decarboxylation the reactant is a carboxylic acid.
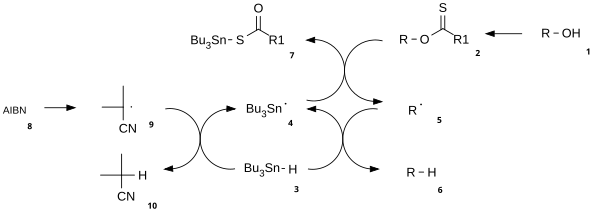
Mechanism
The
Variations
Alternative hydrogen sources
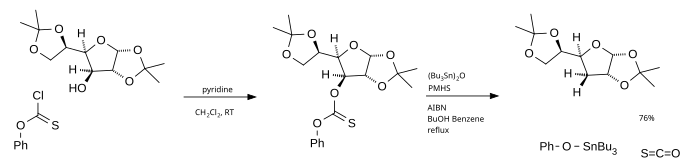
The main disadvantage of this reaction is the use of tributylstannane which is toxic, expensive and difficult to remove from the reaction mixture. One alternative is the use of tributyltin oxide as the radical source and poly(methylhydridesiloxane) (PMHS) as the hydrogen source.[4] Phenyl chlorothionoformate used as the starting material ultimately generates carbonyl sulfide.
Trialkyl boranes
An even more convenient hydrogen donor is provided by
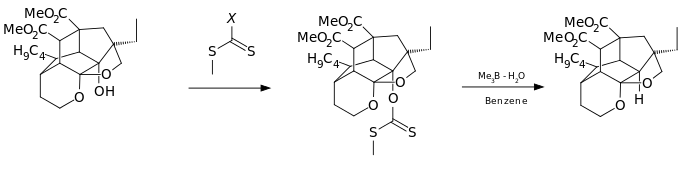
In this

It is found by
Scope
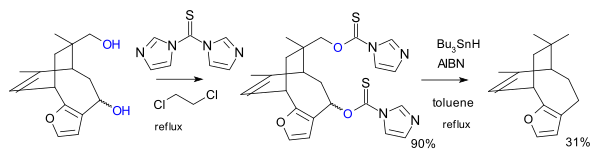
A variation of this reaction was used as one of the steps in the total synthesis of azadirachtin:[6]
In another variation the reagent is the imidazole 1,1'-thiocarbonyldiimidazole (TCDI), for example in the total synthesis of pallescensin B.[7] TCDI is especially good to primary alcohols because there is no resonance stabilization of the xanthate because the nitrogen lonepair is involved in the aromatic sextet.[citation needed]
The reaction also applies to S-alkylxanthates. With triethylborane as a novel metal-free reagent, the required hydrogen atoms are abstracted from protic solvents, the reactor wall or even (in strictly anhydrous conditions) the borane itself.[8]