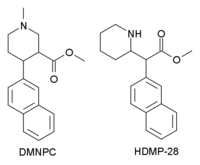
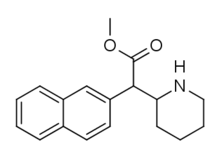
HDMP-28
 | |
| Legal status | |
|---|---|
| Legal status |
|
| Identifiers | |
| |
JSmol) | |
| |
| |
| | |
HDMP-28 or methylnaphthidate is a piperidine based stimulant drug, closely related to methylphenidate, but with the benzene ring replaced by naphthalene. It is a potent dopamine reuptake inhibitor, with several times the potency of methylphenidate and a short duration of action,[1] and is a structural isomer of another potent dopamine reuptake inhibitor, N,O-Dimethyl-4-(2-naphthyl)piperidine-3-carboxylate. It has been sold as a designer drug since around 2015.[2]

Most of the TMP analogs of HDMP-28 have
triple reuptake inhibitor.[3]
| Inhibition of specific analogs at displacing CFT from binding to DAT & RTI-55 from binding to SERT | ||||||
| Ar | [3H] CFT DAT
|
[3H]DA Uptake | [3H]RTI-55 SERT | Inhibition by 10 μM | D.R. | Potency |
|---|---|---|---|---|---|---|
| Ph | 83.9 | 224 | ≫10,000 | 19.6 | 2.7 | 1.00 |
| p-F | 35.0 | 142 | >10,000 | 36.9 | 4.1 | 3.33 |
| m-Cl | 5.1 | 23.0 | >10,000 | 45.5 | 4.5 | 2.42 |
| p-Me | 33.0 | 126 | >10,000 | 45.0 | 3.8 | 0.74 |
| p-NH2 | 34.5 | 114 | ≫10,000 | 7.9 | 3.3 | 2.18 |
| m,p-Cl2 | 5.3 (2.67)b | 7.0 | 1,064 (>10,000)b | 93.3 | 1.3 | 7.98 |
| β-Naphthyl | 33.9b 11.0c | 53.0c | 71.6b | ND | 4.8c | — |
| Cocaine | 160 | 404 | 401 | nd | 2.5 | 0.41 |
| aSchweri, et al. (2002);[4] bDavies, et al. (2004);[5] cDeutsch, et al. (2001).[6] | ||||||
D.R. is the discrimination ratio = [3H]DA ÷ [3H]CFT.
A low D.R. indicates more addictive, whereas a high D.R. indicates low propensity for self-administration.
Legality
HDMP-28 is illegal in Switzerland as of December 2015.[7]
See also
- 3-Bromomethylphenidate
- 3,4-Dichloromethylphenidate
- BMAPN
- Ethylphenidate
- HDEP-28
- Naphthylisopropylamine
- Naphyrone
- 2β-Propanoyl-3β-(2-naphthyl)-tropane (WF-23)
- Isopropylphenidate
- Propylphenidate